US $76
Directions
Similar products from Chemical Supplies

Lithium Carbonate, Powder, Reagent, ACS, 2.5kg

Rhodium metal 99.99% Pure Element 45 Rh Chemistry Sample

Samarium metal 99.9% Pure Element 62 Sm Chemistry Sample

100g (3.52oz) Pure Potassium Iodide Crystal Powder High Purity ACS Grade

Pasco Scientific CI-6734 SODIUM ISE Electrode with Instructions-VG

Spectrum Ethanol, dehydrated alcohol, 200 Proof, 99.9% Undenatured, USP, 120ml
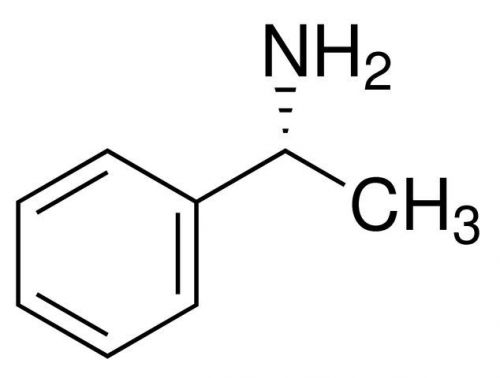
(R)-(+)-1-Phenylethylamine, (R)-(+)-alpha-Methylbenzylamine, 99%, 5ml

Yttrium(III) oxide, reagent, 99.9%, 50g

Biologic Science Instruments RSC-160 Rapid Solution Changer w/ Power Cord RSC160

500g 1.1 lbs Polyvinyl Alcohol (PVA) Fine Powder **CHEAP**
Lithium iodide hydrate, 98.0+%, 25g

5 GALLON NORMAL N. PROPYL ACETATE SOLVENT COATINGS PRINTING INKS

Cobalt(II) Chloride-Hexahydrate Granular - 125grams- 98+% reagent

Cobalt Chloride-Hexahydrate Granular - 10lbs- 98+% reagent

Cobalt Sulfate-Heptahydrate 99.5+% Reagent- Certified- 1Kg
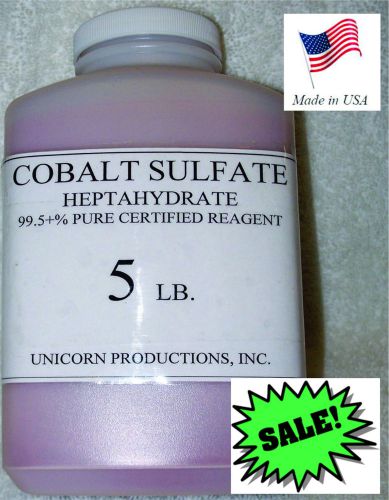
Cobalt Sulfate-Heptahydrate 99.5+% Reagent- Certified- 5 Lbs

Micro-Cide28 HLD 3% Glutaraldehyde MC28-04-128 Liquid

Oakton Waterproof EcoTestr TDS High Tester Pocket Meter New

Liquid Quicksilver Mercury Hg 99.9999% pure 8 oz
People who viewed this item also vieved

pH PAPER ROLL WITH DISPENSER-RANGE 3.0 TO 5.5-UNOPENED-FREE SHIPPING

Micronic M226RP 1.4ml Alphanum printed tubes U-bottom refill Carrier-96 x1728

New Barnstead D8902 HN Ultrapure Mixed-Bed Ion Exchange Hose Nipple Cartridge

Whatman STERILE Syringe Filter GD/X Sterile .2um Pore Size

HEAVY DUTY LARGE SIZE CONDENSER CLAMP THREE PRONG W/ C I GRIP CLAMP Misc Utility

Used 2 Pack Sanitary Nylon Tri-Clamp Laboratory Clamp Measures 1-5/8" Interior

Wheaton Glass Serum Bottles, 20mL or 0.7oz 1 tray of 96 bottles
![CrystalCap Vial - Hampton Research [HR4-904: 60 pack]](/_content/items/images/45/2797345/001.jpg)
CrystalCap Vial - Hampton Research [HR4-904: 60 pack]
![CrystalCap Magnetic (ALS) - Hampton Research [HR4-779: without vial, 60 pack]](/_content/items/images/46/2797346/001.jpg)
CrystalCap Magnetic (ALS) - Hampton Research [HR4-779: without vial, 60 pack]

14mm 19mm Grade 2 Titanium Nail Male Side Arm + Free HoneyCombz Silicone Ball

14mm 19mm Grade 2 Titanium Nail Female Side Arm + Free HoneyCombz Silicone Ball

RAININ PIPETTE EDP PLUS DIGITAL PIPETTE W/ CHARGER Model EP 1000
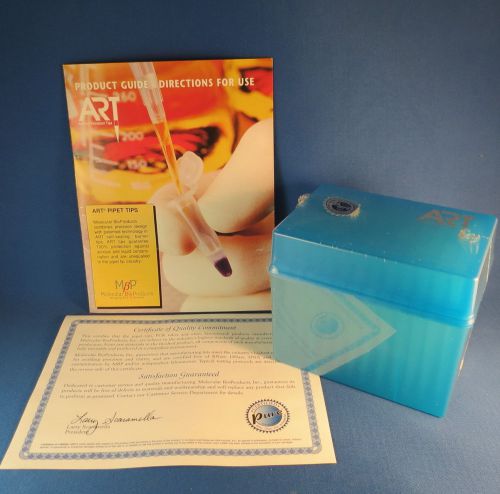
Molecular MBP ART XLG Pipet Tips 200ul # 2160G Qty 768

3 New Fisherbrand Heavy-Duty Utility Funnels 500mL 203mm

Case of 960 Corning Costar 4401 Polypropylene 96-Well Cluster Tubes Well Volume

91Pcs Prepared Glass Student Science Microscope Slides Bio-Microscope Specimen

Vintage Penneys Prepared Slides - Bacteria, Insects, Bees and Butterfly Parts

10 Parker CPI Female Run Tee 4-4-4 MBZ-SS 1/4" Single Ferrule Tube Fitting 316SS

13 Parker CPI 3/8 branch Tee 6-6-4 SBZ-SS 3/8" Tube Fitting 1/4 MNPT NEW
By clicking "Accept All Cookies", you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts.
Accept All Cookies