US $824.95
| Condition | Used
:
An item that has been used previously. The item may have some signs of cosmetic wear, but is fully operational and functions as intended. This item may be a floor model or store return that has been used. See the seller’s listing for full details and description of any imperfections.
|
| Seller Notes | “We acquired this unit from a medical auction and it does not appear to have ever been used. There are a few markings on the case and unit as pictured. All pictures are of the unit and accessories that you will receive. This unit was manufactured in 2011. Please note that the pads and battery will need replacing. The battery does power-up and shows 25% life. The pads in the unit have a "use by" date of 08/2013. Guaranteed to function properly.” |
Directions
Similar products from Automated external defibrillators

WELCH ALLYN AED 20 -, HAS CARRYING CASE-awesome pre-owned

samaritan defibillator sam 300p

Philips HeartStart OnSite Defibrillator Trainer Training AED M5085A bag Free S&h

Zoll M Series BIPHASIC defib, pacing, 12 lead, NIBP, ECG, Masimo SPO2, Bluetooth

Physio-Control LifePak 500 Biphasic ECG EMT Medic EG Great Condition!

Zoll Remote Control AED+ Trainer

Lifepak 500 Hard Shell waterproof case

NEW Philips Heart Start M5066A Home Onsite AED Defibrillator Case Heartstart |

PHILIPS HEARTSTART FRx AUTOMATED EXTERNAL DEFIBRILLATOR

Medtronic Physio-Control 800418 Defib Paddle Pediatric Attachment Child AED

WELCH ALLYN AED Pads for AED 10, AED 20, PIC 30, PIC 40 & PIC 50 -great price!!!

HeartSine AED, Samaritan PAD -300P S/N13C00419764

Model 400-50 Creatron Defibrillator tester

ZOLL AED PLUS | WITH CARRYING CASE

Agilent Tehnologies FR2 Battery Charger M3855A CHARGER ONLY

Philips Heartstart Home Defibrillator M5066A w/ Manual
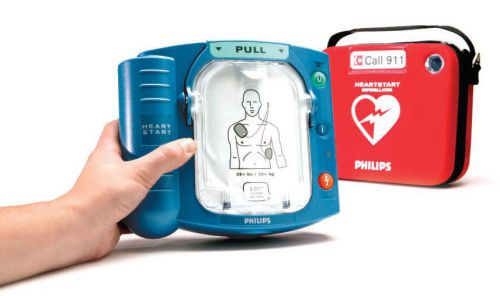
Philips Heartstart Home Defibrillator W/Training Pads and Carry Case

ACTAR CPR MANIKIN LUNGS - PACK OF 100 - ACTAR 911 ADULT/CHILD MANIKIN LUNGS
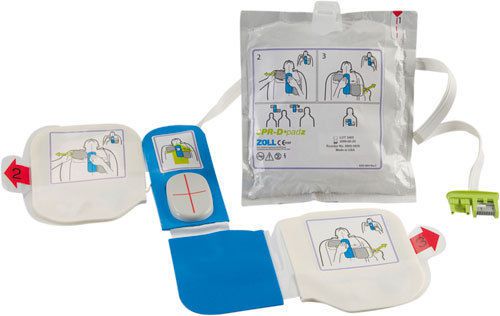
NEW ZOLL AED PLUS CPR-D PADZ Electrodes 8900-0800-01 - 2019 Expiration Date
People who viewed this item also vieved
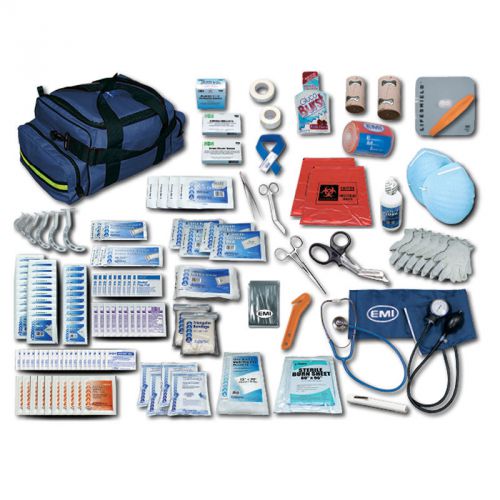
Emergency Medical Technician Pro Response 2 Complete Kit with Navy Bag 1 EA

Emergency Medical Multi Trauma Response Bag - Orange 1 EA

Emergency Medical Technician Pro Response Basic Bag Navy 1 EA

Emergency Medical Technician Rescue Gold Holster Set 1 EA

3 Set Shears; EMT/Scissors combo pack w/holster

New Reusable Aluminum LED PUPIL GAUGE Nurse Pen Light Medical Check flashlight

MedFlash Personal Health Medical Record Storage 1GB (New)

Police Captain Coffee Mug, brand new, full wrap printing

AED Practi-Trainer Essentials CPR Defibrillator Trainer - WL120ES10 AED Trainer
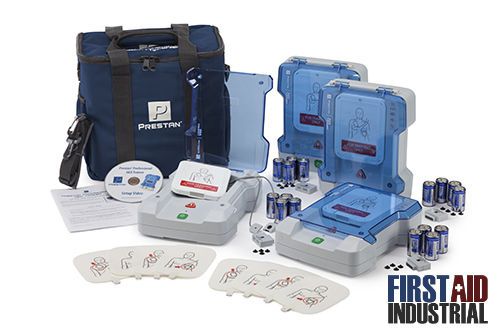
Prestan Professional AED Trainer 4 pack PP-AEDT-401 Defibrillator Trainer

100 pairs/lot Adult training Replacement pads for HR/FR2 AED trainer

Hausted 5E8 Power Eye stretcher pictured working nice condition w hand control

EMS Emergency Rescue Backboard Stretcher

Ferno ProFLEXX 93-P Ambulance Stretcher Cot EMS EMT w/ Black Mattress

Emergency Ambulance Light weight Medical Hospital wheeled Stretcher lift patient
By clicking "Accept All Cookies", you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts.
Accept All Cookies