US $160.00
| Condition: |
New: A brand-new, unused, unopened, undamaged item in its original packaging (where packaging is
applicable). Packaging should be the same as what is found in a retail store, unless the item is handmade or was packaged by the manufacturer in non-retail packaging, such as an unprinted box or plastic bag. See the seller's listing for full details.
...
|
Brand | HYPRO-SORB |
| Country/Region of Manufacture | Czech Republic |
Directions
Similar products from Dental Expendables

Lot of 10 Nobel Biocare Replace Select WP Cover Screws 5.0

Miltex Paste Carriers RA #3 20 mm

Dental Mouth Prop Adult Size. Box Of 2. (F3)

Dental Instrument Set Up Tray - Perfect For WREB

Orthodontic Night Brace Pillow

1 Bag new dental saliva ejector low volume suction green tube disposable 100pcs

Burgundy Yeti Thowax Wax - Sample Size

ClearView Scavenging Circuit 1 w/ Vacuum Control Valve & Single-Use Nasal Hoods
Bilayer Collagen Membrane Hypro-Sorb F - 30 x 40 mm DENTAL Implant Bone Graft CE

20 Packs Dental Ortho Super Elastic Niti ROUND Nature Form Arch Wire 020 lower
new 50pcs/pack Dental Oral Care Oral Floss Flossers ToothPick

1 Kit Dental Orthodontic Ceramic Brackets mesh base Slot Roth 0.022 3 with Hooks

HU-FRIEDY ULTRASONIC INSERT - UNIVERSAL LAVENDER # 10 25K

60pcs Dental Teeth Whitening Cheek Retractor C type 3 sizes small middle and big
3M ESPE Filtek Supreme Ultra Master's Kit (129 capsules - 6029M)

5PackDental ImpressionMaterial Vinyl PolysiloxanePuttyFastSet Base&Catalyst600ml

KAVO,PROPHY PUSH ON ADAPTER FOR PROPHY SCREW IN HEADS,NEW,

Nobel Biocare NobelReplace Tapered Guided Surgery **FREE SHIPPING

Ultrasonic Cleaner ART-UC1 from 1 min. to 99 min Scooba Made in USA by Bonart.
People who viewed this item also vieved

Neytech Qex Vacuum Dental Furnace

Dental Portable Turbine Unit Water Bottle Air Water Syringe Compressor 4 Holes

10Pcs KangQiao Dental Instrument Dental Curettes SR1(round ahndle)

5Pcs KangQiao Dental Instrument Dental Curettes SE1(octagon handle)

12 Dental Slow Low Speed Handpiece Kits Contra Angle Motor Straight Nosecone E-P

8x NSK Style Dental High Speed Handpiece Push Button Type 4 Hole S-Port
Dental Cordless Gutta Percha Obturation Endo System/ Endodontic Pen Gun skysea

Henry Schein Replacement Dental Lamp #100-6347 H3 12V 55W (Lot of 3) 01007

Periodontal Dentoform Kilgore International (2 total)

Dental Spiral Titanium Implant 5.0 x 10 Blister Kit + prosthetic parts + CE FDA

Wired Dental Intra Oral Camera 1/4 Sony CCD/USB/automatic focusing MD830UF
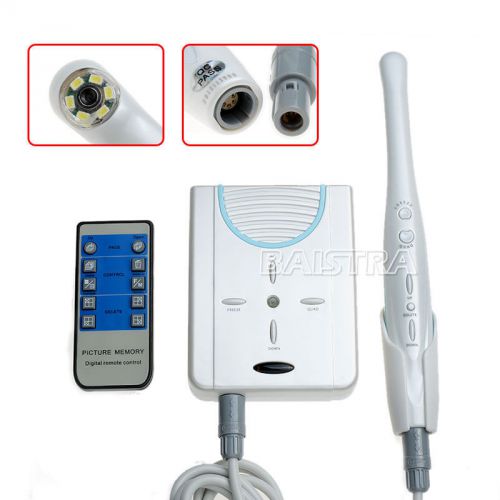
Dental Wired 2.0Mega Pixels 6 LED VGA/USB output Intra Oral Camera MD-910A
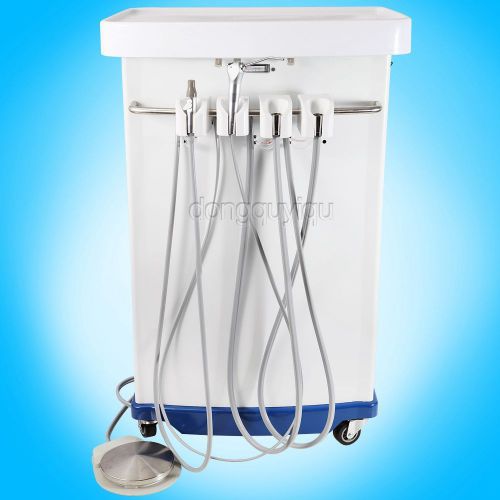
Portable Dental oilless Compressor Self-Contained Delivery Unit Suitcase Dentist

DENTAL EZ DENTAL CHAIR BACK MODEL J,JS, JSA

DENTAL EZ CHAIR BELLOWS MODEL J,JS V, VS, JSA, VSA

UE4 Endodontic Tip Diamond Coated Fit Ultrasurgery Mectron Piezosurgery

5Pcs UE3 Endodontic Tip Diamond Coated Fit Ultrasurgery Mectron Piezosurgery
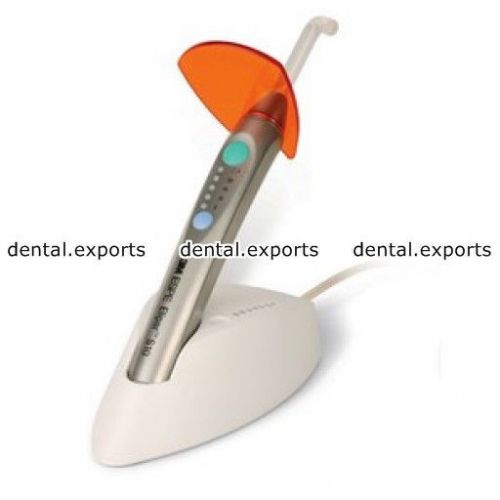
3M ESPE Elipar™ S10 Curing Light

RADIOMETER - CURING LIGHT LED TEST UNIT - DEMETRON/KERR MODEL 100 - MPN 10503

!A! Dentsply Vari-Mix III Dental Amalgamator for Cavity-Filling Amalgam Mixing
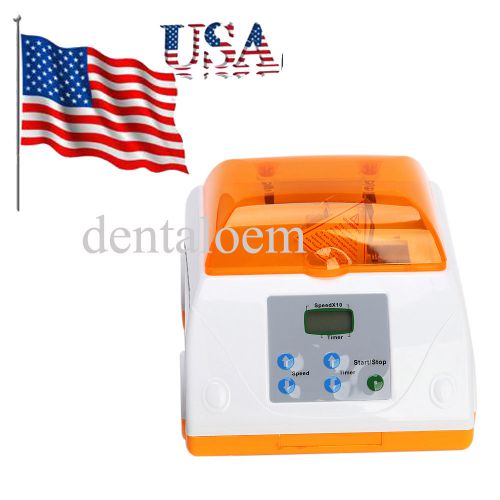
Brand New Dental Lab Equipment Amalgamator Amalgam Capsule Mixer corful choice
By clicking "Accept All Cookies", you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts.
Accept All Cookies