US $1,349.88
Directions
Similar products from Surgical Instruments

Biomet / W. Lorenz Pectus Bar Template

Bausch + Lomb Storz E3855 Grasping Instrument

Biomet / W. Lorenz Pectus Support Bar

2 PCS. SCALPEL KNIFE HANDLE #7
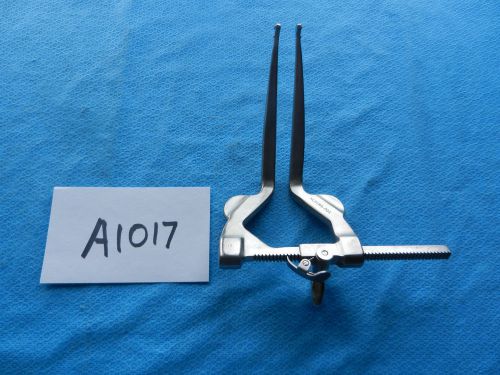
V. Mueller Spine Spinal Collis Lumbar Intervertebral Spreader NL9180-001
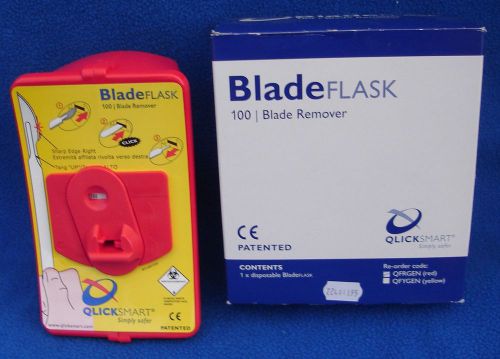
Lot of 3 Qlicksmart BladeFlask Blade Flask - Model ZFRGEN - Red - NEW IN BOX
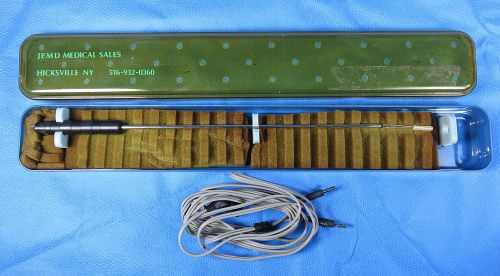
J.E.M.D Myolysis Bipolar Coagulation Needle for Gynecology

FUJI FILM 14x17 ST-VI CR IMAGING PLATE SINGLE PACK ( LOT OF 2 ITEMS )

Coated Vaginal Speculum Gynecology Surgical Instruments
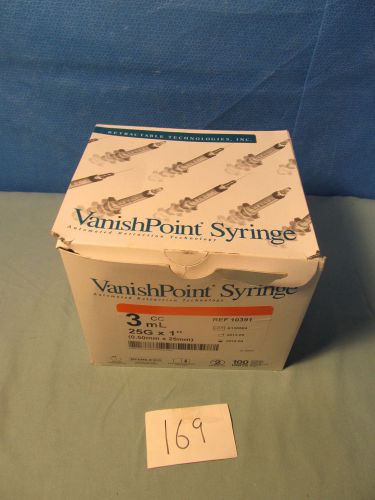
Vanish Point Retractable Syringe 3ml / cc 25g x 1" Ref 10391 (QTY-100)

Alcon Monarch II IOL Delivery System (QTY-5)

Bipolar Endoscopy Assorted Active Cord (QTY-7)

Codman Anterior Fusion Kit 80-1004

I.C.MEDICAL ICM 360 AUTOMATIC SMOKE EVACUATOR @
People who viewed this item also vieved

NEW SCHIRMER Tear Teat Strips Blue Dye 10 Envelopes, 5 Sets Of 2 Strips In Each
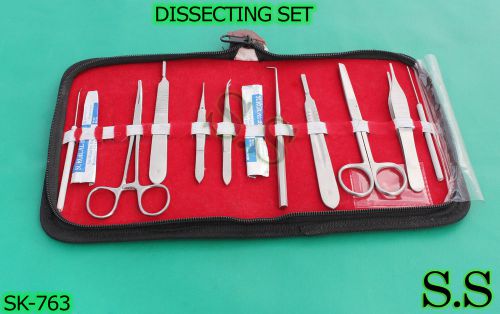
DISSECTING SET - ADVANCED BIOLOGY INSTRUMENTS, SK-763
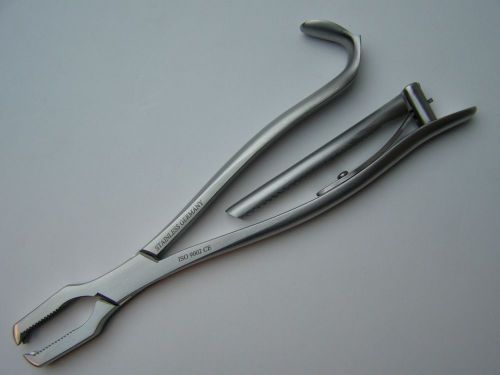
KERN Bone Holding Forceps 7.5" Serrated JAWS Orthopedic Instrument German CE
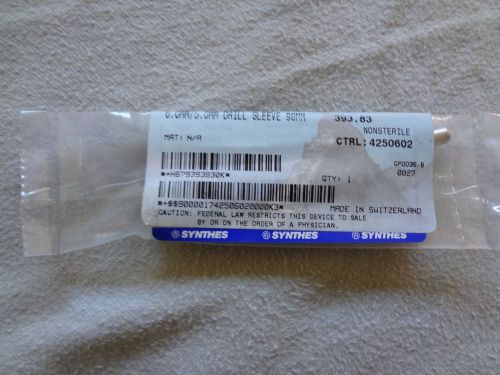
Synthes 6.0mm/5.0mm Drill Sleeve 98mm 393.83

U.S. ARMY Pattern Chiesel 6mm Gouge 8" Orthopedic Instrument German CE

Philips HEART START FR2 AED MODEL M3860A WITH ECG Battery Has LOW Charge!

Easy Vac Aspirator Suction Machine

Chattanooga Digital Hydrocollator M 2 Mobile Heating Unit

Darco Medium Black All Purpose Boot Ref. APB2B

Darco Small Black All Purpose Boot Ref. APB1B

puritan bennett , bdu pcb board

puritan bennett , ai pcb board

CLA Insight Millennium Subluxation Station V9.1.9 All 5 Technologies

Used Chiropractic Adjusting Table

Taktonix GXR-950 IASTM Treatment Chiropractic Activator Table Tool by Miyodac
By clicking "Accept All Cookies", you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts.
Accept All Cookies