US $9.00
| Condition: |
New: A brand-new, unused, unopened, undamaged item in its original packaging (where packaging is
applicable). Packaging should be the same as what is found in a retail store, unless the item is handmade or was packaged by the manufacturer in non-retail packaging, such as an unprinted box or plastic bag. See the seller's listing for full details.
...
|
Brand | OLED |
| MPN | Does Not Apply | ||
| Model | YE | ||
| Country/Region of Manufacture | China |
Directions
Similar products from Pulse Oxymeters
Top Compact Design Single Display Heart Rate Monitor 1.1" OLED Screen SPO2

OLED Blood Oxygen Finger Tip Pulse Oximeter Oxymeter SPO2 PR Monitor 6 modes 50N
CE FDA Daily night sleep Spo2 pulse oximeter Sleep Study PC SW,USB,OLED

PC based Pulse Oximeter/ SPO2 monitor for INFANT Neonatal Baby + USB software

Infant Neonatal baby Handheld Pulse Oximeter Spo2 Heart rate Monitor+ Software

CONTEC Handheld Pulse Oximeter Spo2 PR Heart rate Monitor+ Software +Alarm

Color OLED Wrist Pulse Oximeter-SpO2 Clip Probe Saturation Monitor software CD
CE,FDA Fingertip Heart Pulse Rate Oximeter Monitor Spo2 Oxygen Blood

CMS50ED Finger Pulse Oximeter FingerTip Blood Oxygen SPO2 Monitor OLED display
CE&FDA,Fingertip pulse oximeter, blood oxygen monitor CMS50B, Promotion
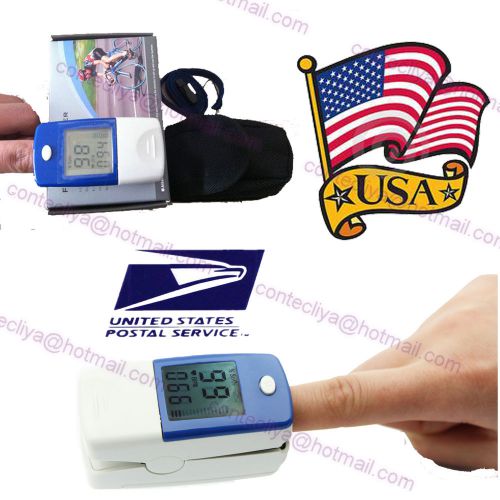
FDA LCD Finger Tip Pulse Oximeter Blood Oxygen SpO2 PR Heart Rate Monitor CMS50B

PULSE OXIMETER B400-1007A ADAPTER CABLE B-4001007A UNBRANDED GENERIC

SENSOR PROBE B505-1015 NOVAMETRIX

COVIDIEN NELLCOR REF: DOC-10 PATIENT MONITORING INTERFACE CABLE

Masimo Rainbow Radical 7 SpO2 Patient Monitor CO Oximeter

Red LNC-1, LNCS SpO2 Spot Check Patient Cable.
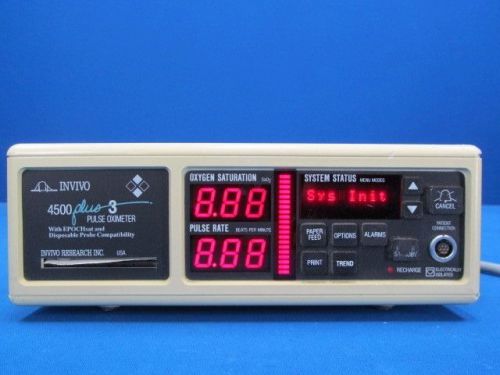
Invivo 4500 Plus 3 Pulse Oximeter* Good Condition

NEW Masimo Red LNC-10 Adapter Patient Cable SpO2 20-Pin 10 Feet Long (READ)

8 New BCI 1303 Infant Pediatric Sensor Disposable Pulse Oximetry Oximeter SpO2
People who viewed this item also vieved

5 Critikon Reusable Dura-Cuf Blood Pressure Cuffs #2774

3 Critikon Reusable Dura-Cuf Blood Pressure Cuffs #2753

9 Critikon Reusable Dura-Cuf Blood Pressure Cuffs #2752

iHealth Wireless Blood Pressure Wrist Monitor

Schiller America cardiovit AT-2/AT-2plus

CE FDA Contec ECG300G Veterinary 3 channel ECG EKG Machine for VET, Pet, Animal
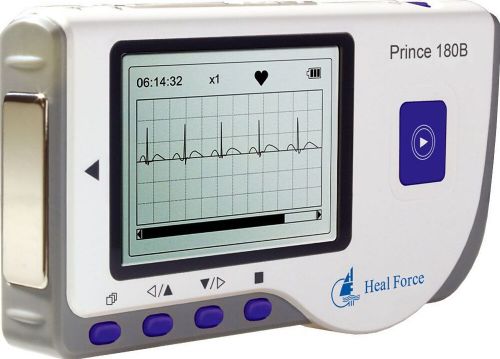
HEAL FORCE PRINCE 180B Portable Easy Handheld Heart ECG Monitor + Software/USB

Schiller 10 lead ekg cable with Multi-functional Limb Clamp Suction Electrodes

High-quality Sony UP-897MD black and white video printer,AV CABLE+1 ROLL paper
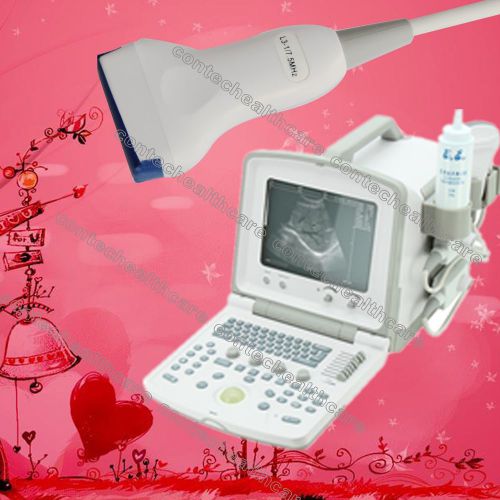
2014 New CMS600B2 Portable B-Ultrasound Digital Diagnostic Scanner Linear Probe

Cas 740-1 Vital signs MAXNIPB, AC Power Supply, Battery & Carrying Case Included

* GRASS COMET XL Polysomnograph Twin PSG/EEG SW & DONGLE KEY

Curbell / Dukane 1801 8 Pillow Speaker

Spacelabs CO2 Sensor 704-0001-00

11141-000028 - LifePak 12 Rechargeable Battery - SLA - Used - working condition

Portable Fetal Doppler Baby Monitors Prenatal Heart Monitors Pink & White & Blue

Portable Fetal Doppler Baby Monitors Prenatal Heart Monitors CE BF-560

BISTOS HI BEBE FETAL DOPPLER PRENATAL HEART MONITOR BRAND NEW IN BOX

Portable Fetal Doppler Baby Monitors Prenatal Heart Monitors CE BF-600+
By clicking "Accept All Cookies", you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts.
Accept All Cookies