US $580
| Condition: |
New: A brand-new, unused, unopened, undamaged item in its original packaging (where packaging is
applicable). Packaging should be the same as what is found in a retail store, unless the item is handmade or was packaged by the manufacturer in non-retail packaging, such as an unprinted box or plastic bag. See the seller's listing for full details.
...
|
Brand | Patient monitor |
| MPN | PM60A | ||
| Model | PM60A |
Directions
Similar products from Health Condition Monitors
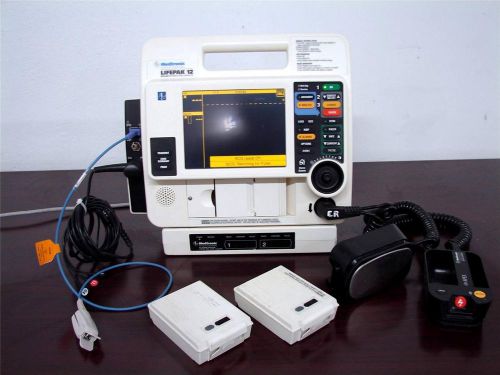
Lifepak 12 BiPhasic ECG NIBP SPO2 Paddles AED Printer 2 Battery AC POWER Charger

Lifepak 12 BiPhasic ECG NIBP W/ Cuff Pacing AED Therapy 2 Batteries Cary case

2010 Lifepak 12 BiPhasic ECG NIBP SPO2 Paddles Pacing AED 2 Batteries Cary case

Datascope Passort XG color monitor W/warranty and leads

Varian MOTOR/POTENTIOMETER ASSEMBLY - CLINAC 18/20 (68.3 RPM). 832400-02

SpaceLabs 90351 NIBP SpO2 monitor module

SpaceLabs 90309 patient monitor w/ psu and ECG, NIBP - SpO2, printer modules

Phillips Intellivue information center 19 inch touch screen monitor

SpaceLabs patient monitor power supply, p/n 119-0479-00

Nellcor Symphony N-3000-N22 SpO2 ECG Patient Monitor w/N-3200-N10 Pulse Oximeter

Marquette Eagle 10" Medical Vital Sign ECG BP Patient Display Monitor PARTS

Datascope Accutorr 3 SAT - Vital Signs Patient Monitor w/ Accessories - WORKS
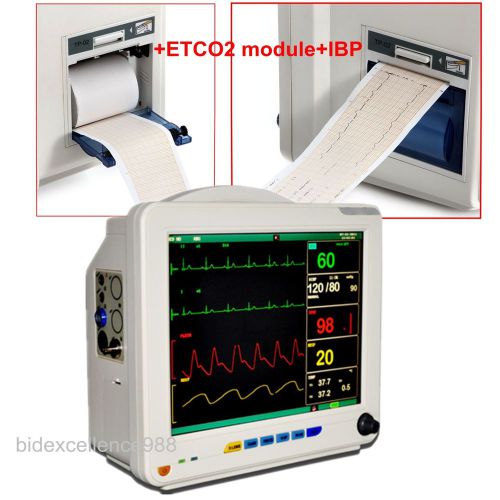
12-inch CCU 6-parameter Patient Monitor+thermal printer+side stream ETCO2 +IBP

US seller ICU Patient Monitor 6 parameter Vital Sign ECG NIBP RESP TEMP SPO2 Pr

Invivo Research AS153 3150 Medical Patient Monitor AC Adapter Power Supply PSU

Medtronic carelink patient monitor

Welch Allyn Propaq CS 242- ECG, SPo2, Pulse,Temp, NIBP And Printer - Nice!!

Edwards Lifesciences Vigileo Patient Monitor 692759-027 w/Pole Mount
People who viewed this item also vieved

Schiller Cardiovit AT-4 Electrocardiograph

Hot!Prince 80B Handheld Electrocardiogram portable ECG EKG Monitor LCD+electrode

NIKOCLIPS EKG ALLIGATOR CLIPS ECG 10/PK

7 inch Color screen Digital 3 channel 12 lead Electrocardiograph ECG-EKG 2Y WARR

Philips Stressvue Medical Stressvue Medical Chart Recorder Thermal Printer

CADWELL CENTRAL EMG/NCV/EP & EEG SYSTEM WITH 1 YEAR WARRANTY

FETAL MONITOR: PHILIPS SERIES 50XM

Nellcor DS-100 Oximax Original Item Used in Demos only

New Arrival Fetal Doppler Prenatal Heart Rate Monitors Eraphone+USB 2colors

2015**Fetal Doppler Prenatal Heart Rate Monitors Eraphone+USB cable Green/Pink

Hand Aneroid Sphygmomanometer Adult Cuff Full Reading Position Model 5098-02

ADC PROSPHYG Proscope Aneroid Sphygmomanometer, Adult 760-11ABKhip

Select Medical SPHYGMOMANOMETER Gauge Adult Cuff Adjustable with Case

Philips M1574A Adult Blood Pressure Cuff NIP

Novametrix Respironics Reusable 9168-00 Superbright Finger Sensor Db-9

Lot of 10 Covidien Nellcor Infant SpO2 Sensor MAXI
By clicking "Accept All Cookies", you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts.
Accept All Cookies