US $1300
Directions
Similar products from Health Condition Monitors

Physio control Hard Paddles for lifepak 6

Lot of Three (3) Zoll AED 8000-1006 Patient Cable

Zoll PD 1200 Monitor ECG Pacing and Cables - working
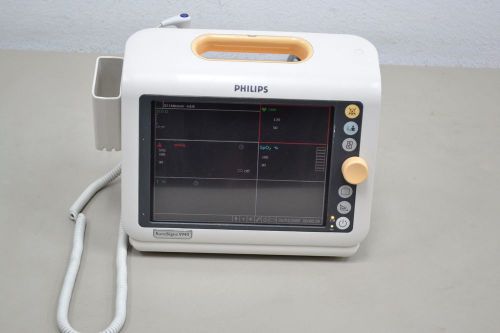
Philips SureSigns VM4 Mobile Patient Monitor ECG SpO2 Temp IBP (11704)

Zoll D 900 Monitor ECG and Cables - working

Nellcor Puritan Bennett NPB-4000 (11705)

GE seer light compact digital holter ambulatory ECG Recorder 5 lead ecg

Qrt 11 of Hospira Micro Macro Plum XL IV Infusion Pump
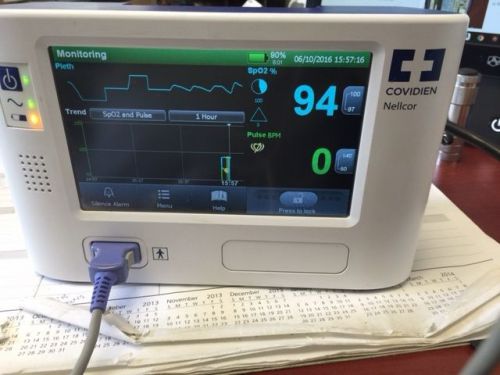
Nellcor Bedside SpO2 Patient Monitoring System GR101704

**Heartrack** Transtelephonic Cardiac Portable Monitor

GE Dash 2000 Vital Signs Patient Monitor (11710)

Agilent M1205A V24C Patient Monitor w/ Modules and Direct Digital Recorder
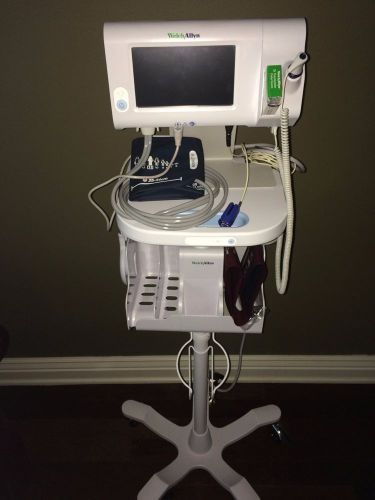
Welch Allyn CONNEX SPOT MONITOR 73WT-B (Bluetooth Connectivity)
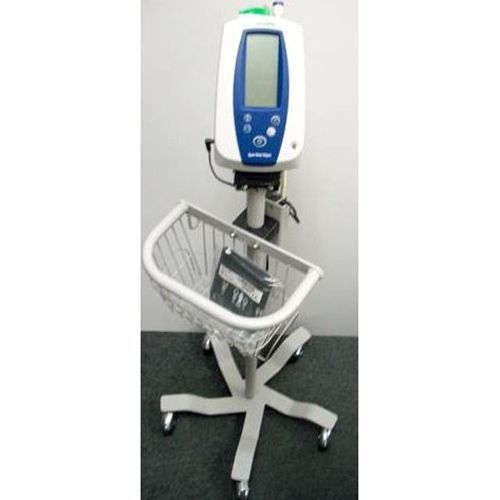
Welch Allyn Spot Vital Signs Monitor *Certified*
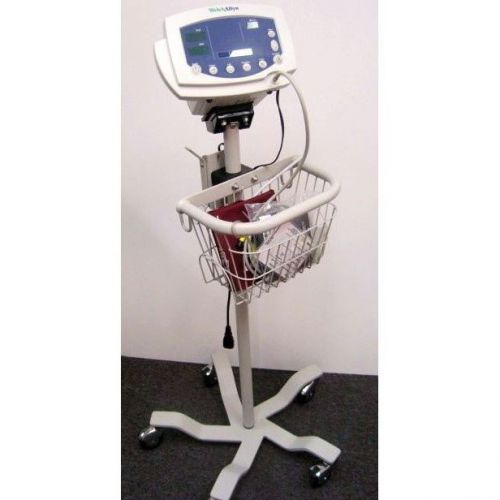
Welch Allyn 53000 Vital Signs Monitor *Certified*
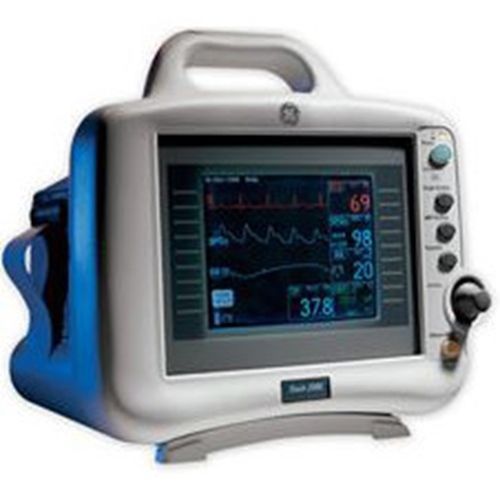
GE / Marquette Dash 2000 ECG Multi Monitor

Zoll Training CPR Stat-Padz Electrodes

Zoll Trainer2 Wireless Remote Controller
People who viewed this item also vieved

Datex-Ohmeda Child Cuff 572426 Lot Of 3
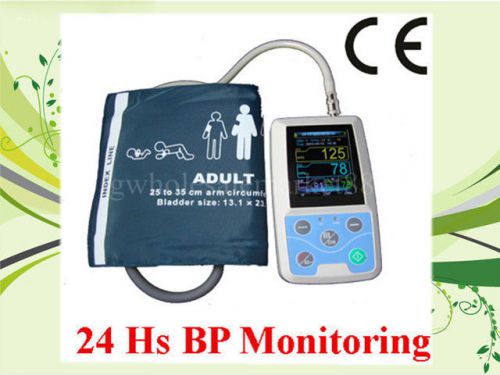
CE 24 hours Ambulatory Blood Press Monitor Holter ABPM + free adult cuff
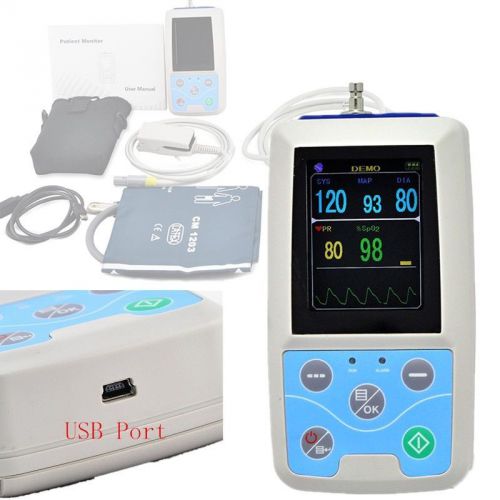
2015 New software Automatic 24h Ambulatory Blood Pressure Monitor Spo2 BP monito

2015 Updated software Ambulatory Blood Pressure Monitor+Automatic 24h+ Spo2 BP

Burdick Atria 6100 EKG (Refurbished)

10leads ECK/EKG calbe for MORTARA RESTING CABLE ELI-150, ELI-210, ELI-250

WELCH ALLYN 08500 REPLACEMENT BULB GENUINE

Healthcare Lighting 35 watt Ritter Exam Light Adj Gooseneck w/ Floor Stand NICE
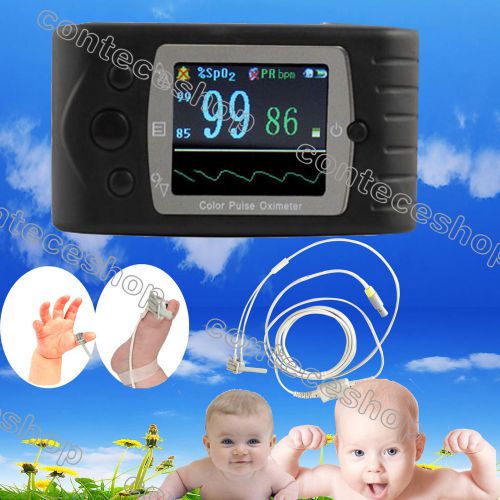
CE infant Finger Pulse Oximeter,SPO2 Monitor,Pulse Rate monitor USB,SW,OLED,NEW

LED Fingertip Pulse Oximeter SpO2 Pulse Rate Monitor,Blood Oxygen Saturation

Hot sale!! Soft black case for CONTEC fingertip pulse oximeter,free rope,lanyard

Medrad MRI SPO2 Pulse Ox Fiberoptic Finger Probe

NONIN Adult Reusable Finger Clip Sensor FOR WELCH ALLYN 5200 FINGER SENSOR

NOVAMETRIX LEMO SENSOR FFA.3S NEW

Dixtal Novametrix SuperBright Finger Sensor REF 8776-00 - NEW
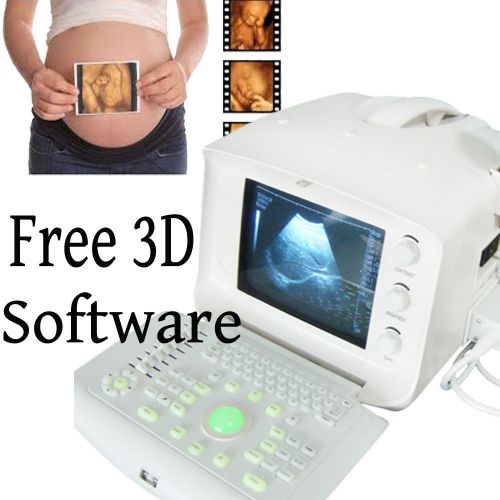
3D KITS Portable Digital Ultrasound Machine/Scanner Convex Linear Transvaginal
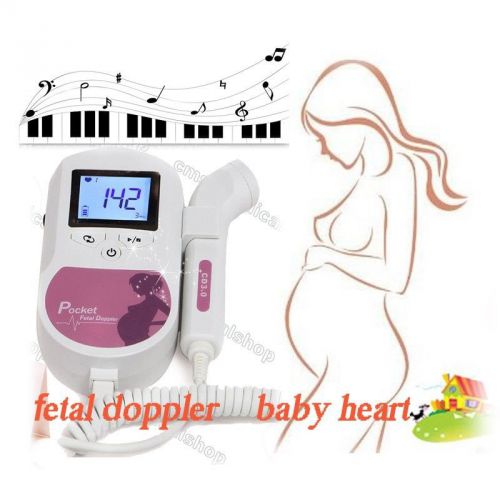
Hot,baby heart monitor,CE&FDA 100% Warranty Sonoline C1,Probe,LCD display,CONTEC

2.5 MHz Probe Fetal Doppler Fetal Heart Monitor with LCD display & Gel

GE COROMETRICS 120 SERIES 0128ANN MEDICAL MATERNAL FETAL MONITOR BP FECG/MECG

Baby Sound B Fetal Doppler, FDA&CE Proved LCD Screen,Free gel,Heart Rate Monitor
By clicking "Accept All Cookies", you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts.
Accept All Cookies