US $4700
| Condition: |
Used: An item that has been used previously. The item may have some signs of cosmetic wear, but is fully
operational and functions as intended. This item may be a floor model or store return that has been used. See the seller’s listing for full details and description of any imperfections.
...
|
Brand | Philips |
| Country/Region of Manufacture | United States | ||
| Model | CT Scanner |
Directions
Similar products from Imaging Esthetics Expendables

PALOMAR STARLUX Pow R 500 HANDPIECE FOR SALE HAND PIECE LASER IPL

Konica Minolta DRY Medical Imaging Film 14"x17" 35 x 43cm SD-PC 35095 M3W8 125
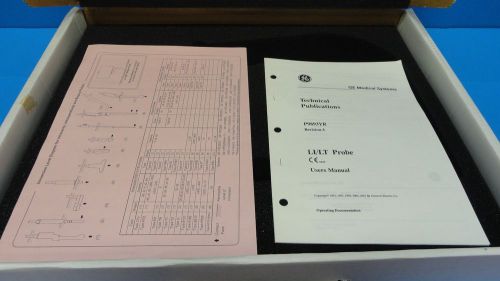
2002 GE LT Linear Array 7.5 MHz Intraoperative / Vascular Probe P/N P9601JB
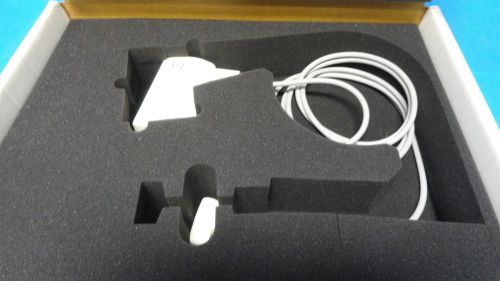
2002 GE 7.5 MHz LT Linear Array IntraoperativeProbe Case & Manual for Logiq 200

SPECTRONICS RADIOGRAPHIC FILM CASSETTE 11` X 14 - 11 PCS

Philips ATL LAP L9-5 Entos Broadband Phased Linear Array Laprasocpic Transducer

ATL BP-TRT CURVED ARRAY BI-PLANE TRANSRECTALTRANSDUCER For ATL HDI Series

ATL AT3C42B / C4-2 Curved Array Broadband Probe (Abdominal OB/GYN General )

Philips L12-5 38 MM Linear Array Probe for Vascular Small Parts Pediatric Breast

2005 ATL L12-5 38MM Linear Array Probe for Vascular Small Parts Pediatric Breast

IMAGING: FUJI CASSETTE 18x24 cm TYPE-CC & ST-VI IMAGING PLATE

ATL C8-5 14R Micro-Convex Pediatric Small Parts Vascular MSK OB Ultrasound Probe

ATL C7-4 40R Curved Array Convex Abdominal Ultrasound Probe for ATL HDI Series
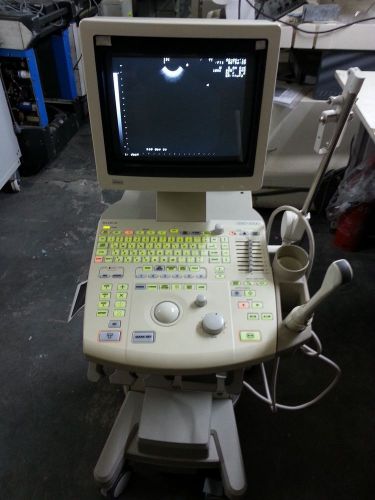
Aloka 1000 Ultrasound Machine no Probe Fully Tested and Patient Ready

ATL Apogee Interspec 2.0 MHz Pencil Non-Imaging Ultrasound Probe W/ Case

KONTRON INSTRUMENTS Trasncranial PW 2.0 MHz Ultrasound Doppler Probe

KONTRON INSTRUMENTS 2.0 MHz Non-Imaging Pencil Ultrasound Doppler Probe

KONTRON INSTRUMENTS CW/PW 2.0 MHz Non-Imaging Pencil Ultrasound Doppler Probe
People who viewed this item also vieved

STRYKER Disposable Electrode ECG EKG Teardrop Foam Resting Tab -ROLL of 90 / NEW

Oxford Varian 300 Nuclear Magnetic Resonance NMR Varian Nice!!!

GE LOGIQ Book Portable Ultrasound Machine

X-Ray Machine Americomp LX125 Collimator Complete Unit Film xray
By clicking "Accept All Cookies", you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts.
Accept All Cookies