US $13000
Directions
Similar products from Other Ophthalmology & Optometry Equipment

Ishihara Book 38 Plates - Vision Testing Book - Ophthalmic Use

White Paddle Ophthalmic Occluder

Lab Retinoscopes Medical Specialties Ophthalmology & Optometry BDN

Optimark Full Diameter Trial Lens Set and Frame in Case. Minus Cylinders (Japan)

Bag of Trial Lens Ophthalmology Trial Lenses with Warranty
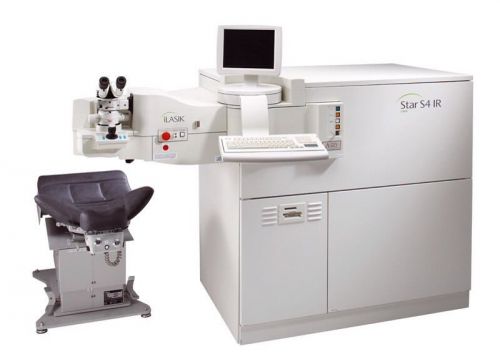
Visx Star4 Excimer Laser with Iris Registration
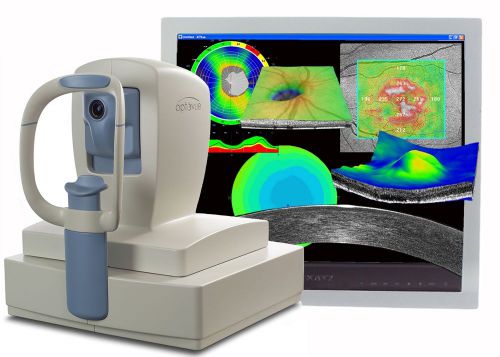
Optovue RTVue OCT - Ophthalmic Equipment

Medical Instrument Bulb - 1209 - 6V25W - New old stock
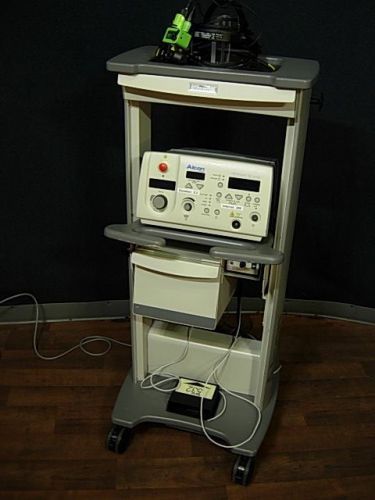
Alcon Surgical Ophthalas 532 Eyelite Laser Photocoagulator

AMO Visx Wavescan Wavefront Custom Aberrometer w Table Printer & Cal Tool WS1

Medical Instrument Bulb - 1460 & 1460x - 6V18W - New old stock

Microscope Bulb - 39-01-86 - 6V50W For Zeiss Microscope & others - New old stock

Hilco PD Rulers Set Of 12 #20-039

Zeiss Visulas 532s with Zeiss 130SL delivery system +/- LIO
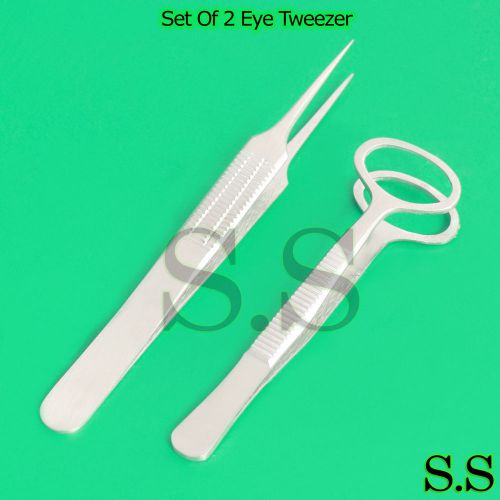
Set Of 2 Eye Tweezer Eye Surgical Instruments

Ocular Iridotomy Sphincterotomy Lens

Oculus BIOM 4pc set with sterilization tray

IRAS Portable Interferometer with Warranty

HUGE Ophthalmic Ophthalmology Parts Lot Haag-Streit Slit Lamp Topcon More! Wow!!

Zeiss F250 Lens Ophthalmoscope Lens Ophthalmic Lens Microscope Lens w/ Warranty
People who viewed this item also vieved

Welch Allyn Panoptic Ophthalmoscope/Otoscope

WELCH ALLYN DIAGNOSTIC SET #97200-MC1 " CLASSIC SET" ALL NEW COMPONENTS!

4X- Ziegler Cilia Forceps Z-2064 - 140

5X- ZABBY'S Cilia Forceps - Wide Z-2056 - 138
By clicking "Accept All Cookies", you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts.
Accept All Cookies