US $520
Directions
Similar products from Other Monitoring Devices
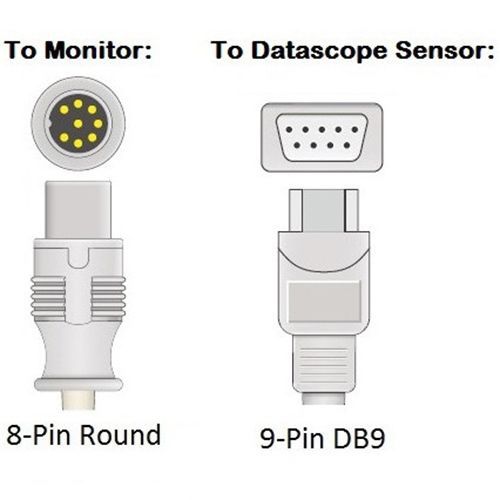
Cables and Sensors Datascope SpO2 Adapter Cable
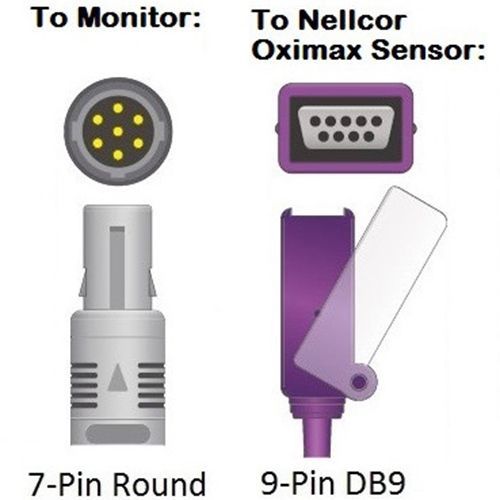
Cables and Sensors Cardell SpO2 Adapter Cable

Cables and Sensors Corometrics SpO2 Adapter Cable

Cables and Sensors BCI SpO2 Adapter Cable

Cables and Sensors Bionet SpO2 Adapter Cable

Bistos Replacement Toco Transducer

B. Braun Disposable IBP Transducer

Acuson 5V2c Adult Cardiac Transducer Ultrasound Probe Sequoia FREE SHIPPING

DATEX OHMEDA GE HEALTHCARE 110-120V POWER SUPPLY F-CU8

GE® Oximax® SpO2 Interface Cable 10ft 2021406-001

Philips M1711A Limb Leads AAMI - Latex Free

St Jude Telectronics 9602 Network Programmer W/ Bag And. Cables
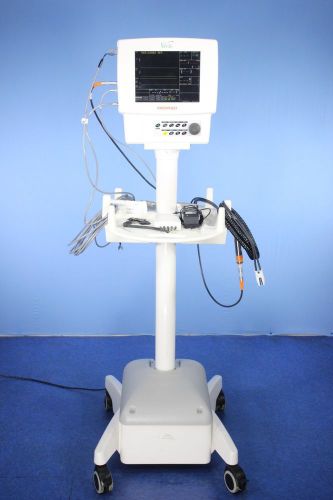
Medrad Veris 8600 MR Vital Signs Monitor MRI Monitor with Warranty
People who viewed this item also vieved
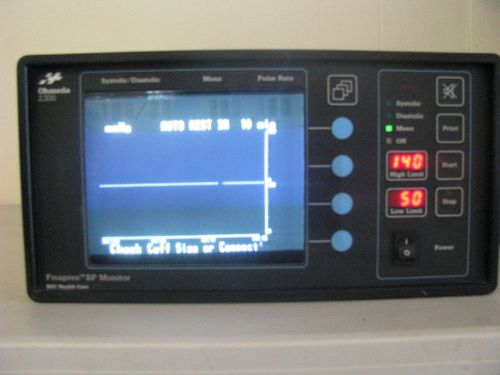
Ohmeda 2300 Finapres Blood Pressure Monitor

MAKE AN OFFER Welch Allyn ProBP 3400 Handheld Digital BP Monitor Device CD ONLY

Welch Allyn DS66 Trigger Aneroid - Gold Series with Adult Cuff

New Welch Allyn CP 200 12-Lead Electrocardiograph ECG CP2A-1E1
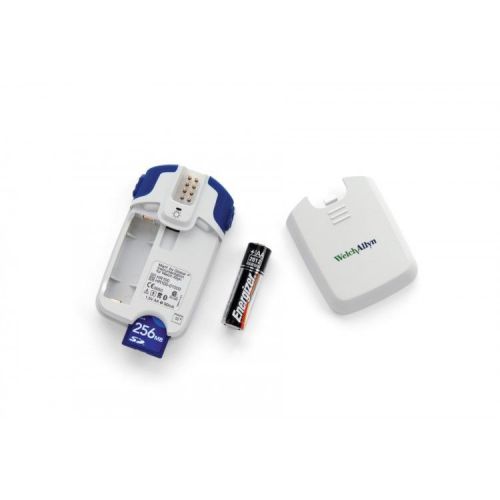
Welch Allyn 401701 Welch Allyn HR-100 Holter Recorder w/ Patient Cable 7Lead
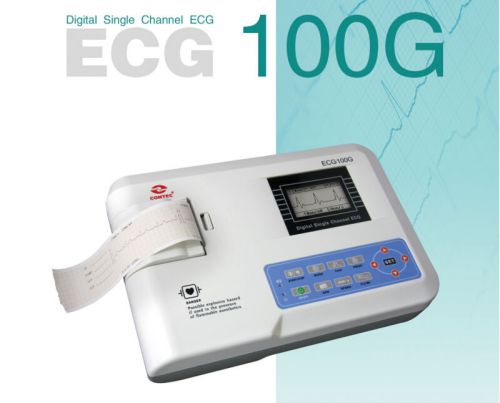
ECG100G Digital ECG EKG machine,Portable Single Channel ECG electrocardiograph

Touch screen 2.83’ color LCD ECG/EKG electrocardiograph 3/6/12-lead ECG system
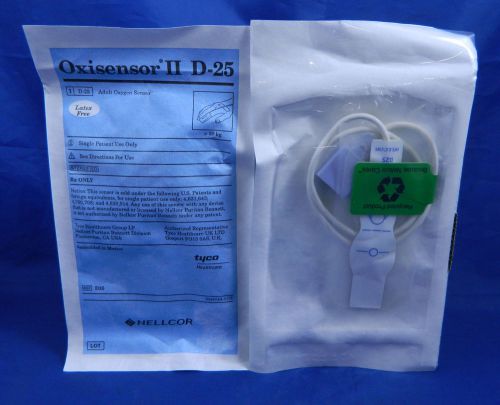
Nellcor OEM Oxisensor II D25 Adult SPO2 Sensor - 24 Pack

Datascope Accusat Pulse OxiMeter

10 Pieces OLED Finger Pulse Oximeter, SPO2 Oximeter, 6 Display,CE FDA Approved

Beep mode!! OLED 5 color Finger Pulse Oximeter, Blood Oxygen, PR, SPO2 monitor

Raytheon Medical Systems G140706

Raytheon Medical Systems G140702

Raytheon Medical Systems G140602
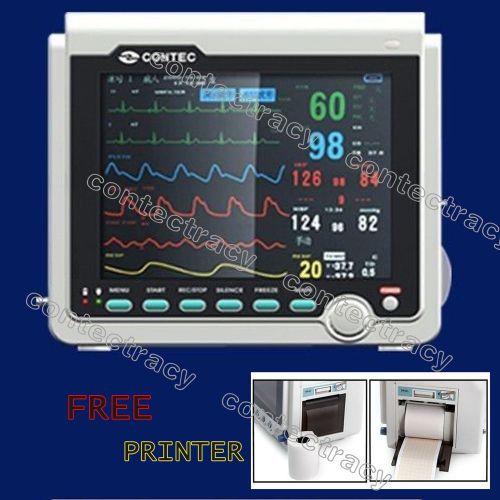
CE Patient Monitor ECG,NIBP,Spo2,Resp,Temp,PRINTER,8.4"colour,Portable monitor

Oxford Instruments Sonicaid Model #121 Fetal Monitor Hand Doppler! Prenatal Look
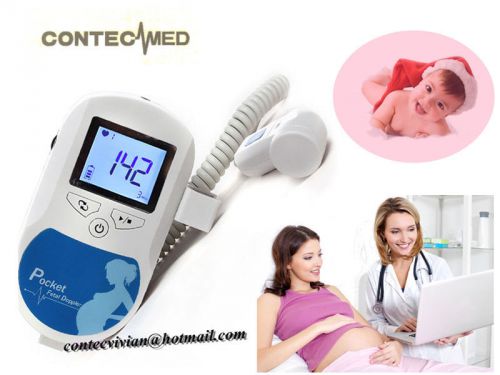
3MHz Prenatal Heart Monitor Fetal Doppler SONOLINE C1 LCD Backlight w Warranty
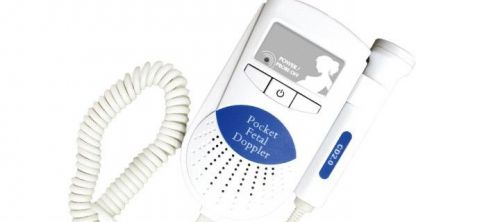
FDA/CE Sonoline A Pocket Fetal Doppler 3Mhz Baby Heart Beat Monitor (NO LCD)
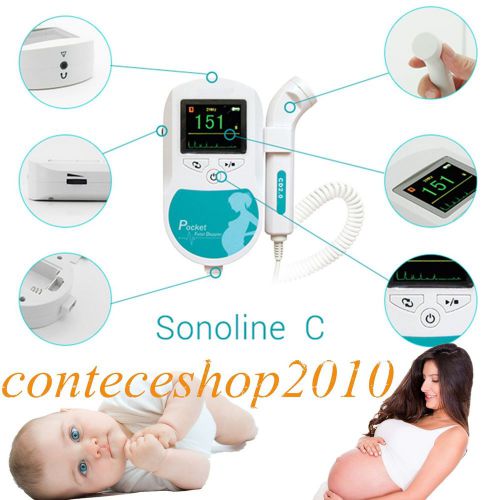
Color LCD Sonoline C 3.0 MHZ probe fetal doppler,baby heart monitor,FHR CE
By clicking "Accept All Cookies", you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts.
Accept All Cookies