US $2500
Directions
Similar products from Other Medical Devices & Machines
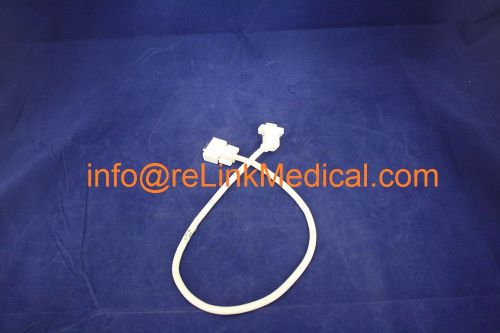
51444572 GE Healthcare COLLISION SENSOR CABLE - LC2
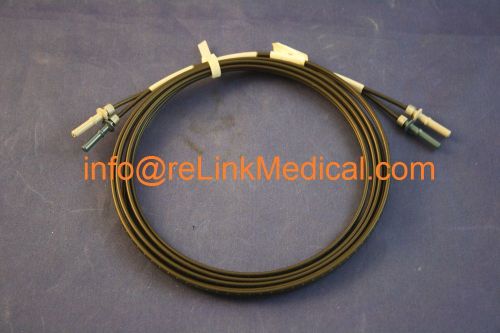
2259288 GE Healthcare FIBER OPTIC CABLE

SANDHILL SCIENTIFIC Z00-2000 SLEUTH REFLUX DETECTIVE / RECORDER + ACCESSORIES !!

2259286 GE Healthcare FIBER OPTIC CABLE ANGIO/INNOVA

Siemens Magnetom Vision Extremity Coil MRI 1597249 (untested) / ME22
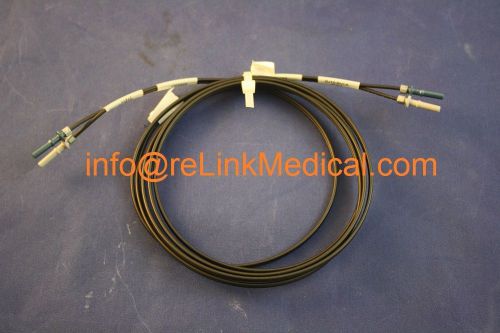
GE Healthcare FIBER OPTIC CABLE INNOVA

Syneron Velaspray Ease treatment spray

CHAD DRIVE Bonsai 800 Series Oxygen Regulator OM-812 New in Box and Also M6 Bag

1707 Grason-Stadler Audiometer with Telephonics TDH-50 headphones

LOT OF 2 KARL STORZ 28124 ARTHROSCOPY SHEATHS ARTHROSCOPIC ! K936

ELMED ARTHROSCOPY SHEATH ARTHROSCOPIC ! K908

LOT OF 3 PN 377-031-530 STRYKER ARTHROSCOPY SHEATHS ! K939

Parks Medical Ultrasonic Doppler Flow Detector Model 812

STRYKER 4.5MM PN 275-724-120 AND 275-724-100 ENDOSCOPY ! K987

KCI Prevena 125 portable negative pressure wound therapy unit

V. MUELLER #GL3211 ROCHESTER-PEAN HYSTERECTOMY FORCEPS - LOT OF 2

Lot of 2 Reliant ABC-3520 Oxygen Regulator 50psi o2
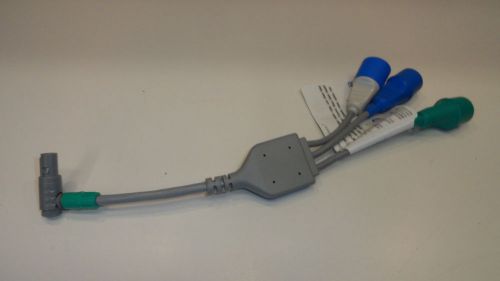
SS11: Edwards LifeSciences EV1000 EVFTC1 Flotrac Cable

Edwards Lifesciences Pressure Monitoring Holder Clamp DTSC New In Box
People who viewed this item also vieved

Lot of 2 Welch Allyn AM232 Manual Audiometer

Eseco Speedmaster SM-14 Pocket Pal Densitometer
Rubbermaid 23 Gallon Step-On Mobile Medical Waste Can, Beige (RCP 6146 BEI)

HOT SALE!!! CMS50C Oximeter Fingertip Pulse Blood Oxygen SpO2 Monitor CE&FDA

2 Behind Ear BEST Sound Amplifier Deaf Hearing Aid Aids EASY OPERATION Digital

GE Healthcare Remote Controller Control 9375-0047-004/C PKD CDA 2199947/0

B Braun 490043 Infusomat Space Pump Y-Type Blood Set (24 per cs)

Alaris IVAC MedSystem III 2860 Series Infusion Pump - Multi Channel IV Pump

Baxter AP II Ambulatory PCA Infusion Pump 2L3105R
By clicking "Accept All Cookies", you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts.
Accept All Cookies