US $765.00
| Condition: |
Used: An item that has been used previously. The item may have some signs of cosmetic wear, but is fully
operational and functions as intended. This item may be a floor model or store return that has been used. See the seller’s listing for full details and description of any imperfections.
...
|
Model | Index 2 |
| Product | Electrosurgical Generator | ||
| Country/Region of Manufacture | United States | ||
| Brand | Fluke |
Directions
Similar products from Manikins & Dummies for Training

CPR Prompt Training Manikin Set 5 Adult With Duffel Bag And Accessories

Infant CPR Anytime Kit AHA Heart Association DVD Training + Baby Manikin

USA MS400 Touch Screen Handheld ECG Simulator Multi Parameters Patient Simulator

CONTEC MS200 NIBP Simulator Non-Invasive Blood Pressure simulation Simulator USA

Adult CPR Mannequin Dark Skin Healthcare Training Doll Torso Flexible Head
Simulaids 2069 African-American Kate/Beth Mouth piece in 10-Pack - b

Learn CPR with Family and Friends CPR Anytime DVD Kit American Heart Association

Learn CPR With Family and Friends CPR Anytime Mini Manikin DVD Kit

Laerdal Resusci Anne Torso CPR Medical Manikin Training In Case ~ S5977

240 Vials - Wallcur Practice-Midazolam Simulation Medication Versed - Demo Dose

Resusci Anne Replacement Manikin Laerdal CPR Face Training - Lot of 10 pieces
PRESTAN Adult Series CPR Manikins + Mouth/Nose Replacements + Lung Bags
CPR PROMPT CPR TRAINING EMT ADULT MANIKIN MANNEQUIN HEAD ONLY
Prestan Child AED CPR Manikin (w/ Monitor) Med Skin PP-CM-100M-MS

Laerdal FST1908 VitalSim Vital Signs Simulator w/ FST1909 Handset Case & Manual

LAERDAL RECORDING RESUSCI ANNE FULL BODY FIRST AID CPR TRAINING MANIKIN
Medical Manikin Training First Aid Nursing Trainer Torso Only
Simulaids 101-102 Interactive ECG Rhythm Simulator Box Training EKG ACLS

Family & Friends CPR Anytime KIT Manikin & DVD American Heart Association - NIB!
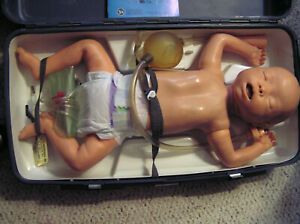
Laerdal Resusci baby Infant cpr training manikin and case
People who viewed this item also vieved

Laerdal Heartstart 3000 Defibrillator AED ECG EKG Patient Heart Monitor

Laerdal Heartstart 3000 ATS Defibrillator AED ECG EKG Patient Heart Monitor

Medtronic Paddle Holders Carrying Case EMT pouches
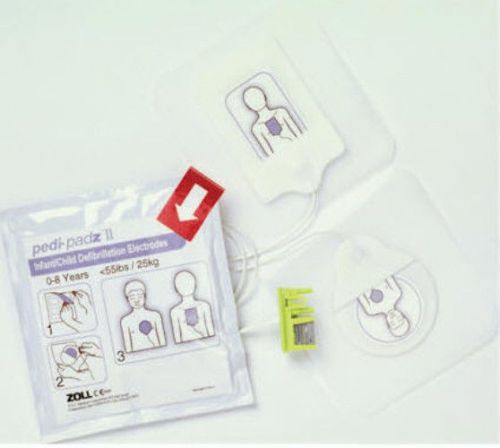
Zoll 8900-0810-01 Pedi-Padz II Pediatric Electrodes for Zoll AED Plus

Condor Outdoor Rip Away EMT Pouch Tri-Fold Design MA41-002

EMS Traction Device Like Kendrick Traction Device KTD Optimum OTD Emergency
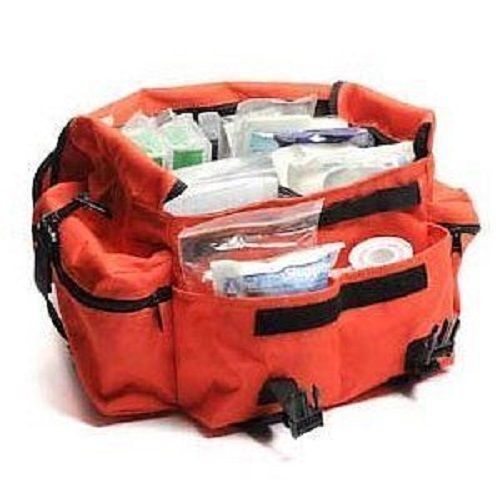
First Responder Trauma Bag Fully Stocked EMT Aid Kit Gunshot Firefighter Police

M17 Medical Bag First Aid Survival Prepper Family Live Emergency Life

5 Each, Military Issue, H&H Inc., "H"-Bandage, Compression Dressing(a)
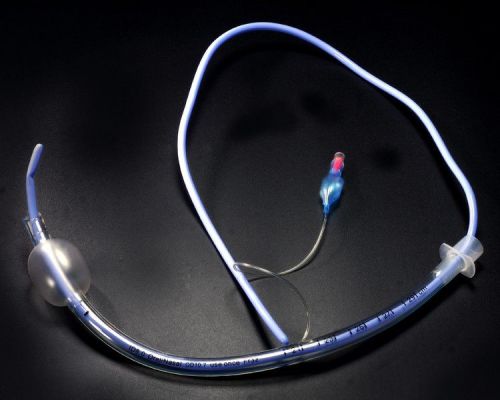
Bougie for Endotracheal tube intubation Adult and Pediatric

BRAND NEW IV START KIT IV STARTER I.V. KIT

SKLAR 7 1/2" UTILITY SCISSORS TRAUMA EMT EMS BLUE SERRATED 11-1280

Amulance Stretcher Lockdown Rail

Ambulance Stretcher Side Rail for Stryker-RECONDITIONED

Steris Hausted APC All Purpose Chair
By clicking "Accept All Cookies", you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts.
Accept All Cookies