GBP 999.99
| Condition: |
Used: An item that has been used previously. The item may have some signs of cosmetic wear, but is fully
operational and functions as intended. This item may be a floor model or store return that has been used. See the seller’s listing for full details and description of any imperfections.
...
|
Country/Region of Manufacture | Finland |
| Monitor Parameters | CO2 | ||
| MPN | S/5 S5 Carescape Patient Oxygen O2 N2O Anesthesia | ||
| Brand | GE |
Directions
Similar products from Health Condition Monitors
Philips SureSigns VS3 Patient Monitor

BIS Aspect Medical System Monitor Model A-2000

Datascope PASSPORT 2lt Patient Monitor ECG MASIMO SpO2 NIBP Printer recorder

Datascope Duo Patient Monitor Lot Of 6
Medtronic 24960 MyCareLink Relay Home Communicator
8'' Portable Medical Patient Monitor Machine Vital Sign ECG,NIBP,SPO2,TEMP,PR

2 Invivo Precess 3160DCU MRI Patient Monitor, Module Battery Charger 3 Batteries
New TruSignal Ear Sensor From GE

SECURE CAS-SPT Caregiver Alert System Wireless Sensor Pad Transmitter - NEW/FAST

Nihon Kohden ZS-940PA Telemetry Medical Transmitter
PHILIPS INTELLIVUE X2 M3002-60010 VITAL SIGNS MONITOR FRONT PANEL

Mindray Datascope Accutorr Plus Vital Signs Monitor with NIBP Hose Rolling Stand

Mindray Accutorr 7 SmarTemp Temperature Probe - Same Day Shipping

Burdick Treamill Patient Box 007772 used. With aquisition cable
Cheetah Medical Nicom Reliant Patient Monitor w/ Cables

8" ICU CCU Vital Sign Patient Monitor 6 parameter ECG NIBP RESP TEMP SPO2 PR

Color Portable ICU CCU Vital Signs Patient Monitor ECG NIBP RESP TEMP SPO2 PR

Lifepak 12 / Biphasic / Monitor / for parts only

GE TruSignal SpO2 AllFit Sensor TS-AF-10 Lot of 5~Fast Free Shipping!
People who viewed this item also vieved

Electronic Sphygmamonometer Blood Pressure Monitor CONTEC08A

BAINBRIDGE Sphygmomanometer with Welch Allyn Blood Presure Cuff-Adult, New !!!
Contec Automatic Blood Pressure Monitor, Grey, ABPM50

Blood Pressure Adult cuff Arden/Philips with Clippard air hose 12 feet

WELCH ALLYN SCHILLER AT-2 PLUS EKG ECG W/ SCHILLER SP-250 SPIROMETRY

NIHON KOHLER NEUROMASTER MEE-1000A ELECTRODES, MANUALS & SOFTWARE

GE MarquetteMulti-link trunk Cable with leadwires 22341809+38401817 MAC-1200

Adult Finger Clip Spo2 Sensor Probe Round 10 Pin Fit Datascope S/5,AS/3,CS/3,Car
OLED Fingertip oxymeter spo2,PR monitor Blood Oxygen Pulse Oximeter CE YK80

Nonin WristOx2 Pulse Oximeter with starter kit (Model 3150SK)

BRAND NEW PHILIPS SIDESTREAM CO2 SENSOR MODULE

Nonin Neonate Wrap SpO2 Sensor 7 pins 3ft Compatible

GE/Dukane Telligence Station Gateway Brand New

Stryker 5921-044-135 Color Cuff Tourniquet Cuff
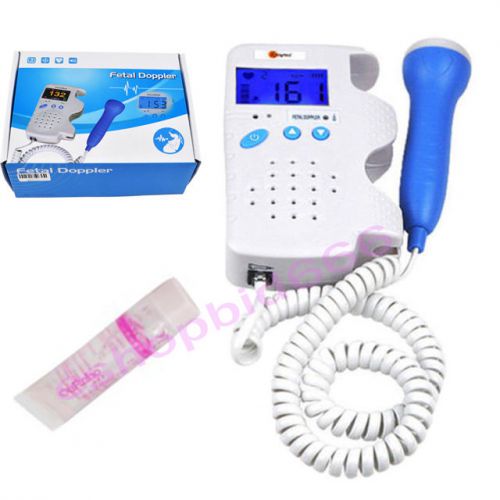
New FDA Fetal Dopper baby heart monitor+Gel with Sound for pregnant woman use CE
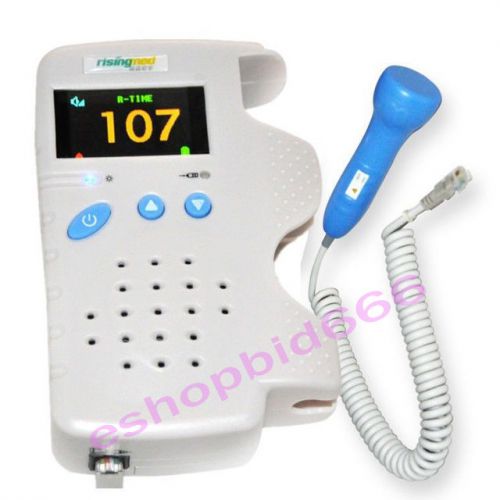
2014 Fetal Doppler 3MHz Color LCD Back Light Heart Beat Waveform Pregnant Baby
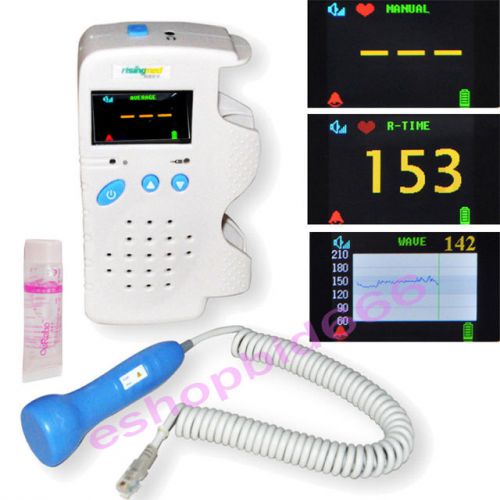
Pregnant Fetal Doppler 3MHz Color LCD Back Light & Heart Beat Waveform + gel A++
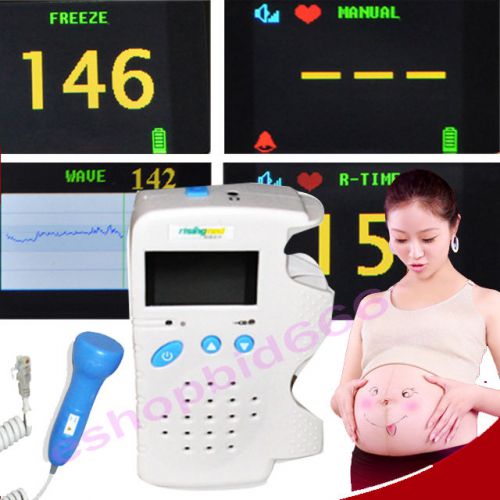
CE pregnant Fetal Doppler 3MHz Color LCD Back Light & Heart Beat Waveform + gel
By clicking "Accept All Cookies", you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts.
Accept All Cookies