US $29.00
Directions
Similar products from Chemical Supplies

100 grams (3.52 oz) High Purity 99.999% TUNGSTEN W Metal Bead #EY4A

100 grams (3.52 oz) High Purity 99.999% TUNGSTEN W Metal Pillar #EY4B

100 grams (3.52 oz) High Purity 99.999% TUNGSTEN W Metal Pill #EY4C

Potassium Nitrate 400g Top Quality Material 99.8% Purity

10 KG DOW Chemical Carbowax PEG Polyethylene Glycol 8000 Granular 25322-68-3

OAKTON 00654-04 Buffer Solution,pH,7.00,500 mL

Cole Parmer Ph Meter Electrode 59001-65 Single Junction-gel filled,epoxy body

Manganese(II) Sulfate Monohydrate 1lb (450 grams) MnSO4·(H2O) FREE SHIPPING

Copper (II) Oxide, Cupric Oxide 1lb (450 grams) FREE SHIPPING

Copper (II) Carbonate, Basic 1lb (450 grams) FREE SHIPPING
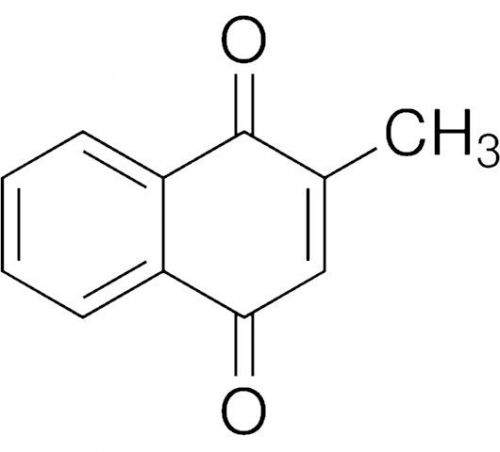
Vitamin K3 VK3 Menadione Pharm Lab Vet Horticultural Grade 99.11% Pure 100 GRAMS

Copper(II) Chloride Dihydrate 1lb (450 grams) FREE SHIPPING

Sodium Nitrate 1lb (450 grams) NaNO2. FREE SHIPPING

Sodium Alginate 1lb (450 grams). FREE SHIPPING

100g Sodium Borohydride NaBH4. FREE SHIPPING

Sodium Bromide 1lb (450 grams) NaBr. FREE SHIPPING

Sodium Stearate 1lb (450 grams). FREE SHIPPING

HANNA INSTRUMENTS HI98302 DiST 2 TDS Meter B0085WUNZ2

Phosphorus Rare Element Sample 1g 99.9% Pure

Molecular sieve 3A Reagent 500g
People who viewed this item also vieved

- Personal Protection Organizer 22.375"W x 5"D x 19.875"H 1 ea

- Isolation Station 16.5"W x 4.25"D x 25.25"H 1 ea

Corning Pyrex Clear Borosilicate Glass Rod 31.7mm (1 1/4 in) - Total of 5 Pieces

Bell in vacuum jar sound physics demonstration demo water boil air pressure New

LOT OF 3 SWAGELOK 7/8" OD x 3/4" MALE NPT CONNECTOR BRASS B-1410-1-12 NEW

7mm*5mm OD 7mm ID 5mm PTFE TEFLON Tubing Tube Pipe hose/5m 5meter NEW

Vollrath Stainless Steel Ware Tray or Box with Lid #8283 - Medical

LOT OF 6 3M C31 LARGE COMMERCIAL INDUSTRIAL SPONGE (C17-2-21)

Lot of 9 Vintage Thomas Steel Lab Spring Clamps

Lot of 4 Vintage RING STANDS For Chemistry Lab Humbolt Cast Iron

Canliners LOT 60 gal trash bags rubbish 2 mil and 0.9 mil X7658QK and H7658TK

REXAM 200 ml reagent vessels 42 pcs

New HPLC column Tosoh TSKgel SuperSW3000 4.6 mm x 30 cm 4 um TSK gel 18675 SW300

New HPLC column Tosoh TSKgel G2000H HR 7.8 mm x 30 cm 5 um TSK gel 17353

25 BD Falcon 353263 Polypropylene NonSterile 96-Well Assay Plate 250µL V-Bottom

25 VWR 89004-367 Prestilised 50mL Graduated Conical Centrifuge Tubes w/Screw Cap

Vintage 1960's Clay-Adams Dissecting Kit

NEW Lab-Aids 5 55 Piece Cheese Making Study Harmless Bacteria Kit 30 Students

2 DRUMMOND PIPET-AID XP PIPETTES

Gilson Pipetman Pipette P200 Adjustable Volume 20 - 200 ul #10 Small top

Prepared Textile Fiber Microscope Slides Set of 12
Square Microscope Cover Glass Slide Slips 100pc 18mm aei-50
By clicking "Accept All Cookies", you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts.
Accept All Cookies