US $29.00
Directions
Similar products from Chemical Supplies
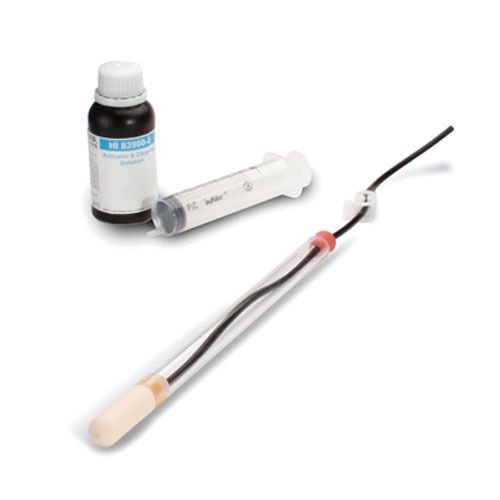
Hanna Instruments HI83900-30 Suction Lysimeter, 30cm w/ Solution Kit

Sodium Thiosulfate, ~1000g Sigma-Aldrich 99%, 217263
![1-Acetyl-1H-1,2,3-triazolo,[4,5-b]pyridine, Aldrich 5g, 34,727-2](/_content/items/images/73/2791773/001.jpg)
1-Acetyl-1H-1,2,3-triazolo,[4,5-b]pyridine, Aldrich 5g, 34,727-2

5-Oxo-5-phenylvaleric acid, 4-benzoylbutyric Acid, Aldrich, 10g b12687

Sodium Dichromate dihydrate, ~300g ALdrich 23,929-1

AD-Mix-Alpha, Aldrich, 392758, 50g

Copper Sulphate - Dry Crystals 4 Ounces In Plastic bottle (Copper Sulfate) USA

Cobalt(II, III) oxide, powder, reagent, 99.5%, 10g

5-Sulfosalicylic acid dihydrate, reagent, 99.0+%, 100g

Rhodium Chloride (RhCl3) Black Powder 41.80% Rhodium 109g

Ruthenium Chloride (RuCl2) 50 grams Sealed

Iridium Sodium Chloride Hexahydrate (Na2IrCl6*6H2O) 23.5 grams Dark Gray Powder

20 KG BOX - VIVAPUR® MCC 101 Microcrystalline Cellulose 100% Pure Powder NF,Ph,

ZINC SULFIDE (ZnS)99.99% PURE- 2mm-13mm Pieces ( 20kg=20,000g )

Cobalt Metal Powder- from pure broken cathodes, sub 325 mesh, H2 annealed- 1lbs
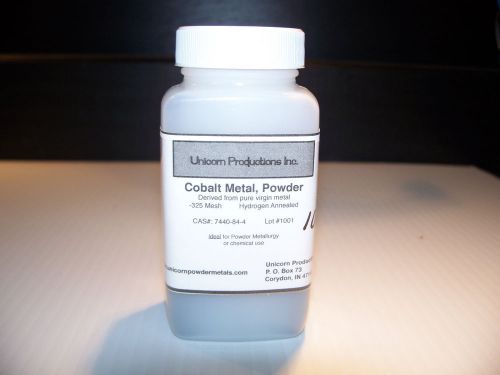
Cobalt Metal Powder- from pure broken cathode, sub 325 mesh, H2 annealed- 100gms
People who viewed this item also vieved

Dupont Tychem SL Chemical Resistant Apron Coat Sz Large White

ENCON EYEWASH STATION - GRAVITY FED PORTABLE - ANSI CERTIFIED MODEL Z358.1-2009

Lot of 4 Ace Glass Condensers (400mm 8 bulb Allihn, Air Cooled Vigreux, Leibig)

213B2 Karter Scientific Glass Erlenmeyer Flask 5 Piece Set 50 150 250 500 New

Fisherbrand Cylindrical Infrared Quartz Cuvettes, 20mm Path-L, 6.3ml,?18.5mm

New Swagelok Fittings!! 3/8 Unions, Elbows, And Tees!!

Swagelok SS, 3/8" Ferrules (30 pcs)

25 Imm. 4 Flt Culture Plate Cat No. 0110103855 All Used with Covers

6 Beckman Skatron A/S Racks with 96 slots #265272
40 Place Transparent Test Tube Rack Holder 18mm holes
Aluminium Anodized Racks 50 tubes 12 mm

50 - Cellucap Disposable Lab Coat Jackets 6512EWHL 40N249 White XL Latex Free

Globe Scientific 116152B Polyethylene Flange Plug Cap for Test Tubes, 16mm Size,

Lot of 5 NEW Millipore CDFC01204 Super-C 12" PE Matrix Carbon Filter Cartridge

Whatman 10497502 Microbiological Membrane Filter Monitor, 47mm Diameter, 0.45 Mi
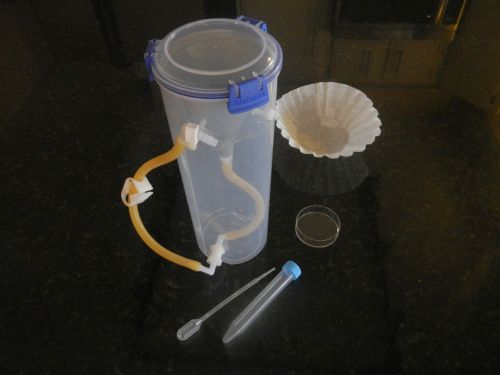
Lungworm test kit. An improved Baermann funnel enclosed to reduce odors.

Antique Filament Light Bulb Radio Style

Lot of Plastic Liquid Pipette Tips Autoclavable

G2Plus G2PLUS? 100PCS 3ml Disposable Plastic Graduated Transfer Pipettes

EISCO Eisco Labs Watch Glass (Beaker Cover), 7.5cm, Pack of 12

(5) Surgipath 00210 White Precleaned Micro Slides SnowCoat X-Tra 1" x 3" x 1.0MM
By clicking "Accept All Cookies", you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts.
Accept All Cookies