US $20.00
| Condition: |
New: A brand-new, unused, unopened, undamaged item in its original packaging (where packaging is
applicable). Packaging should be the same as what is found in a retail store, unless the item is handmade or was packaged by the manufacturer in non-retail packaging, such as an unprinted box or plastic bag. See the seller's listing for full details.
...
|
Brand | Marquest |
| MPN | REF 60350 |
Directions
Similar products from Feeding & Infusion Pumps

Baxter AP II Ambulatory PCA Infusion Pump 2L3105R

Alaris IVAC MedSystem III 2860 Series Infusion Pump - Multi Channel IV Pump

B Braun 490043 Infusomat Space Pump Y-Type Blood Set (24 per cs)

B Braun 490037 Infusomat Space Pump Low Adsorption Set (24 per cs)

Hospira Lifecare PCA Syringe Pump

Excelsior Syringe Pump (ESP)- Excellent Working Condition

Fenwal Blood Component Recipient Set Blood Filter 4C2160 (QTY-Lot of 7)

Carefusion Alaris 20 Drop Secondary Set 72213N - LOT of 20 !!

5 Kimberly-Clark MIC-KEY 30cm Bolus Extension Set REF: 0123-12 Use By 03/2017

Alaris 20 Drop Secondary Set 72213N (QTY-Lot of 79)

Hospira Abbott Plum A+ Single Channel Infusion Pump
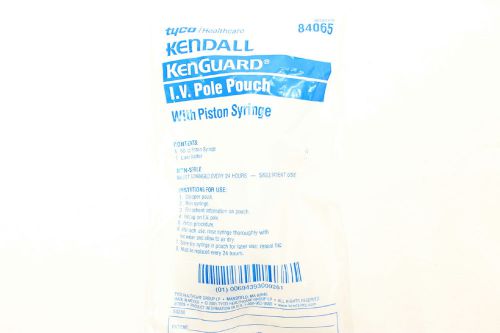
Tyco Kendall Kenguard IV Pole Pouch w/60 cc Piston Syringe 13 ct Healthcare
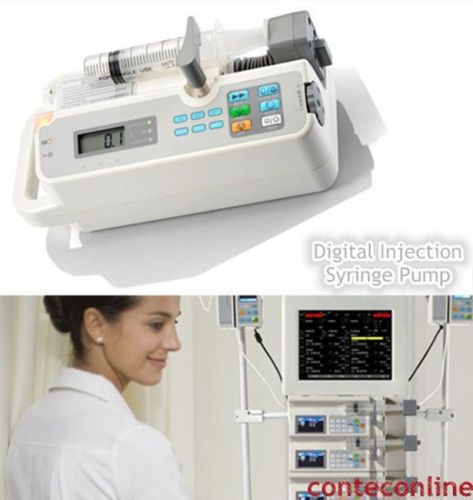
NEW !!! CONTEC Digital Injection / Syringe Pump, Perfusor Compact Pump, SK-500I

GLOBTEK 12 Volt AC Adapter Power Supply for Infusion Dynamics IV Pump NEW

Level 1 Hot Line HL-90 Fluid Warmer

Baxter I-Pump Pain Management System 2L3217
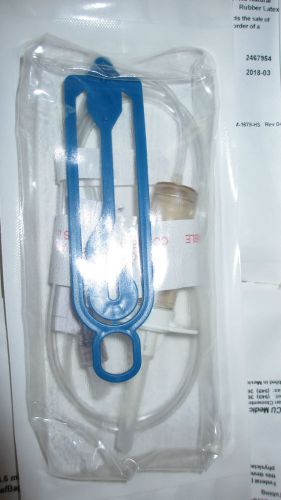
TWO(2) B3162 ICU MEDICAL 41" 4.6 ML 15 DROP ADMIN SET ROTATING LUER w/BAG HANGER

2014 NEW Version Medical + alarm Full Digital <ml/h or drop/min > Infusion Pump
People who viewed this item also vieved

Pro Healthy Weight Loss Body Shape Slim Cavitation Vacuum Lipo Laser Cellulite

Siemens Acuson Keyboard Control Panel for Sequoia Ultrasound System PARTS #1

Acuson MX3-R Multiplexer Plug-In Board for Siemens Sequoia 512 Ultrasound Sys #1

Pulmonetics sprint battery pack for Carefusion LTV series vent free shipping
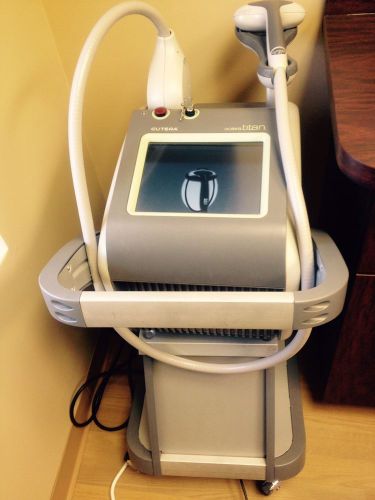
2011 Cutera Solera Titan System

STERIS P764326-484 REPAIR KIT, EXHAUST MANIF 266492

Sani Cloth Plus Germicidal Disposable Cloths Expired 04/14 Kills HPV HIV Germs 6

NEW MTS Micro Needle Therapy Anti-Aging Complex System

Precision EasyGoVac PM65 Medical Aspirator w/ Nonfunctional Power Adapter

Acupressure Wrist Band Magnetic Bracelet With 5 Free Shujok Rings
By clicking "Accept All Cookies", you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts.
Accept All Cookies