US $34,990.00
| Condition | Used
:
An item that has been used previously. The item may have some signs of cosmetic wear, but is fully operational and functions as intended. This item may be a floor model or store return that has been used. See the seller’s listing for full details and description of any imperfections.
|
| Seller Notes | “Working,when de-installedAS-IS WHERE IS -MIAMI FLORIDA 33166” |
Directions
Similar products from Imaging Esthetics Devices

Sonosite M-Turbo C60x curved linear transducer

Portable 10W LED Cold Light Source Charge Dock Storz Wolf Olympus Connection

VARIAN/ Philips Paxscan 2020 PET Camera / Scanner Command processor MPN:37174

Philips 6203 CC 20' oc display #452213184341 CONRAC GMBH

Amber Enterprises AE-4256 Pro-View Professional Imaging System / Infrared Camera
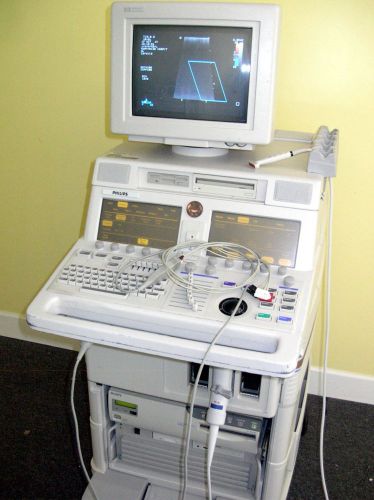
Philips M2424A Sonos 5500 Ultrasound With 15-6L Probe And EKG Leads

Konica Minolta Regius Sigma 2 CR System - 45 Plates / hr - w/ cassettes

KODAK ULTRA-SPEED DF56 DENTAL FILM

Toshiba MinXray-300 Portable X-Ray Machine Vet Veterinary

ATL ULTRAMARK 9 ULTRASOUND, 5 TRANSDUCERS

2 EIZO Eizo FlexScan S2100 21.3" High End LCD Monitors w/ Anthro Mobile Cart
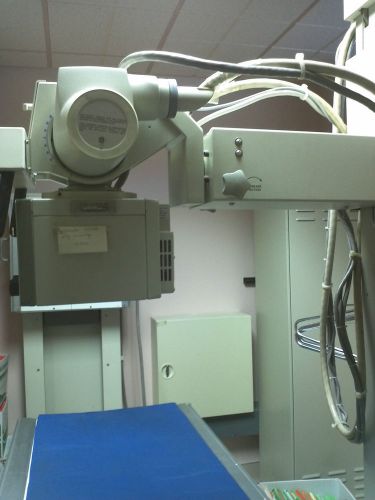
X-Ray Machine- Picker CLINIX-R Imaging, Rad Room, Year 1991, MPN 3962

Dermaveiw Skin Analyzer Model 200

ACUSON SEQUOIA C512 ULTRASOUND WITH TRANSDUCERS

AGFA DRYSTAR 2000 X-RAY FILM PROCESSOR WITH AGFA LINX PASSPORT

NEW* Midmark Dental Procenter Over Patient Delivery System 153600-009 Chair unit

2005 Cynosure Apogee Elite Laser with SmartCool 5

Biolitec Ceralas D-25 25Watt 980nm Surgical Laser

GENERAL ELECTRIC AMX3 MODEL 46-217900-G2
People who viewed this item also vieved

Parker Laboratories Thermasonic Gel Warmer Model 8204

2 Nuclear Associates Victoreen Dual Color Electronic Sensitometer Model 07-417
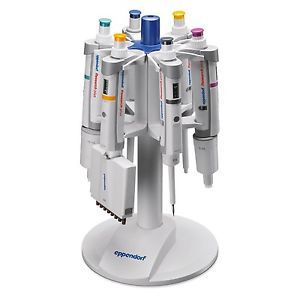
Eppendorf 022444905 Pipette Carousel Rack Stand For Six Pipettes

Philips Central Monitor System Transformer for 4 ohm Speaker P/N 453564046781
By clicking "Accept All Cookies", you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts.
Accept All Cookies