US $2100
Directions
Similar products from Other Equipment for Endoscopy & Laparoscopy

Olympus Endoscopy System Interface Cable MAJ-200

Storz 723620H Thumfart Suction and Irrigation Handle Without Pressure Valve New

Arthrex SCB Image 1 HD Camera Console, HD H3-ZA Camera, Xenon 300 Light Source +

DYONICS SMITH & NEPHEW INC. ED-3 3 CHIP MEDICAL VIDEO CAMERA HEAD ! (134377)

GENUINE OLYMPUS OTV-S6 CAMERA CONTROL UNIT + CAMERA TESTED WARRANTY
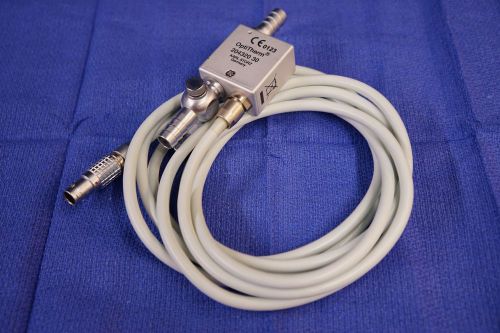
Storz 20432030 Optitherm Heating Element for Thermoflator Endoscopy Laparoscopy

Olympus A2912 Dual Valve Cysto Sheath & A0404 Blind Obturator, 19.8Fr

Olympus A4510 Urethroscope Inner Sheath A4511 Outer Sheath 22Fr with Obturator

Olympus A2796 Single Channel Telescope Bridge 12Fr Endoscopy Laparoscopy.
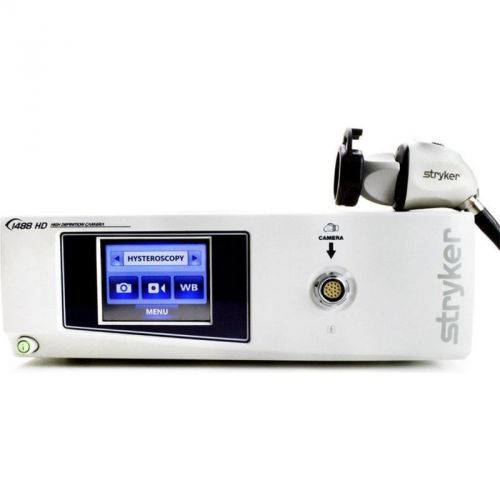
Stryker 1488 HD Camera System *Certified*
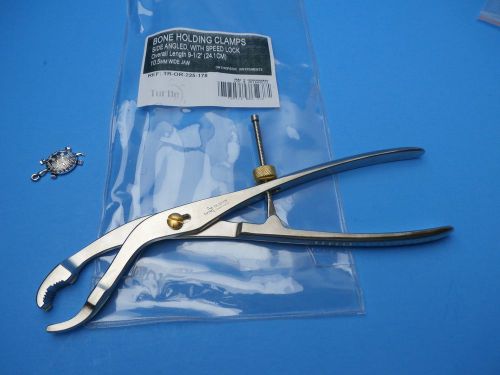
BONE HOLDING CLAMPS 24.1cm-Turtle#TR-OR-225-178,Orthopedic Instruments,OR-GERMAN

Stryker 988 Medical Video Camera *Certified*

Storz Telecam SL Endoscopic Camera System *Certified*

Storz Telecam Endoscopic Camera System *Certified*
Storz 7230 CA Hopkins Lateral Telescope 70° *Certified*

ACMI CIRCON Inner Sheath ERIS-CF25 & Outer Sheath 25.6Fr EROS-CF25 w- Obturator

BONE HOLDING CLAMPS 26cm -Turtle#TR-OR-225-179,Orthopedic Instruments,OR-GERMAN

Olympus A2761,Workingelement A2642 Inner Sheath & Outer Sheath A2624 Endoscopy

Richard Wolf R.Wolf 8382.02 Surgical Straight Laparoscopic Scissor Instrument

Olympus A42011A Cysto Inner Sheath 8mm with A22081A Obturator 24Fr Endoscopy
People who viewed this item also vieved

Coherent Ocular OSMGA Single Mirror Gonio Laser Lens "Must See" !$

CYF-4 Flexible Fiber Cystoscope

Custom Ultrasonic 83-2 C.I. Endoscope Washer Ultrasonic Cleaner Endoscopy

Storz Germany Hopkins BX 29020 A Endoscopy Periscope Endoscope Extended Warranty

Pilling 52-1160 Fiber Optic Light Guide Cable NEW

CODMAN MICROSYSTEM TWIN BEAM LIGHT SOURCE WITH HEAD LAMP *

Stryker X800 XENON Light Source In Great Working Condition

FUJINON FIL-150 ENDOSCOPE LIGHT SOURCE

Karl Storz 22260131-3 DCI-D1 IMAGE 1 Single - Chip Camera Head, NTSC, CE

Olympus Keymed ETO Sterilization Case Endoscopy Endoscopic Endoscope

Man & Machine Really Cool RCK G2 Wired Keyboard waterproof Medical

Olympus A2498 Bridge with single port working channel

Arthrocare REF# EIC4835-01 ENT Coblator II with Intergrated Cable (Reprocessed)

Smith & Nephew Dyonics 72200616 PowerMax Elite Arthroscopy Shaver

Synthes REF# 359.207 BAR FOR SMALL F-TOOL

Synthes REF# 357.367 135 DEG AIMING ARM F/TROCHANTERIC FIXATION NAILS

Stryker Vision 19" Monitor Model 240-030-900 Strykervision Flat Panel Monitor

Stryker 240-050-888 SDC HD High Definition Digital Video Capture Device

Olympus HYF 1T Flexible Hysteroscope
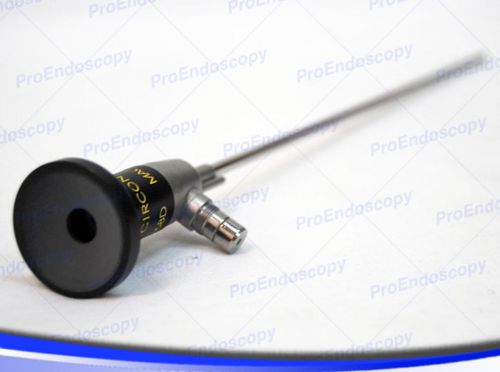
ACMI FO 8168D Cystoscope 4mm 0 degrees
By clicking "Accept All Cookies", you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts.
Accept All Cookies