US $150
Directions
Similar products from Chemical Supplies

Silk Amino Acids PF - For external use only - 1 pound

Lavender Hydrosol - For external use only - 1 pound

Alfa Aesar 43092 Poly(allylamine hydrochloride) Powder. Lot: P25B004.

Sodium Metasilicate 15 Lb Consists of 3- 5 Lb Packs

Chromium(III) potassium sulfate dodecahydrate, Chrome alum, reagent, 98.5%, 100g

Sodium Lauryl Sulfate (SLS) Usp/Kosher 25 Lb. Pack Consists of 5- 5 Lb Packs

Sodium Metasilicate 25 Lb Pack Consists of 5- 5 Lb Packs

Sodium Lauryl Sulfate (SLS) Usp/Kosher 15 Lb. Consists of 3- 5 Lb Packs

BORIC ACID, Amresco 500 gms 0588-500G

Vintage 1950's Gilbert #20 Copper Sulfate Jar 1.75" Tall

Potassium hexacyanoferrate(III), Potassium ferricyanide, reagent, 99,0+%, 50g

Thermo Scientific Orion IonPlus NITRATE ISA Ionic Strength Adjuster 475mL 930711

Orion ROSS Storage Solution 810001 Thermo Scientific 11/2017 475mL / 1 Pint

General WK7200 Complete Waterproof Self Calibrating Micro Processor

ALUMINUM metal POWDER 1 lb Pound 99.9% Lab Chemical

Thermo Scientific Orion® AC45ST Primary Turbidity Calibration Standards Kit

Limited! 1 Lb Aqua regia chemical supply kit! Gold refining kit!!

Hematoxylin & Eosin Stain kit or H&E Stain kit, (250ml/bottle)

Hematoxylin & Eosin Stain kit or H&E Stain kit, (15ml/drip bottle)
People who viewed this item also vieved

30mL SURE Seal universal plain label plastic vials in vitro use only

Sigma P-2241 Anti-c-Myc HS, Coated Plates 96-Well (Clear) New Lot of 10 OEM Pack

Erlab Captari F4AS Type Replacement Carbon Fume Hood Filter NIB

Lot of 2 Thomas Pinch Clamps #18 A

Lot of 2 Thomas Pinch Clamps #28

CYLINDERS GRADUATED MEASURING 5mL 25mL LAB BOROSILICATE GLASS 5 25 mL NEW

Beaker Set of 600mL 250mL 100mL & Cylinder Set of 250mL 100mL 50mL Lab Glass New

Respirator Bag - Full Mask (5EA)

2-pc Fleece/Neoprene Balaclava (12EA)

New Eppendorf PCR tubes 0.2ml,1000 pack

SYPRO Protein Gel Stain Starter Kit S-12012

300ul 12 CHANNEL (ITEM# 443 /4)

Axygen Pipet Tips T-350-C-R, 300uL, 1Unit

NALGENE 5 1/2 GAL/20L RECTANGELAR CARBOY WITH STAINLESS STEEL HANDLE

Nalgene 6L 2240 Jerrican Carboy High Density Polyethylene HDOE

Swagelok Cajon Whitey Plug Valve B-4P4T 1/4" Brass 1/4 Turn Valve

Kobold SV-6204 Flowmeter 41R57 SPST Switch New AMAT Spare
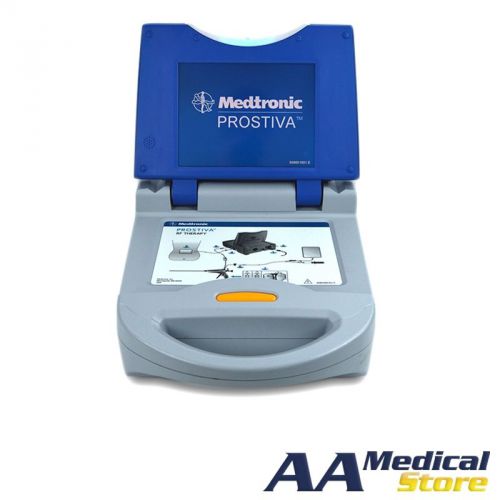
Medtronic 8930 PROSTIVA RF Therapy Console
Labpaq Dissecting Kit, Skeleton Model, and LabPaq CD

Microscope Slide Box, White, 25 slide capacity

Microscope Slide Box, White, 100 slide capacity
By clicking "Accept All Cookies", you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts.
Accept All Cookies