US $28.00
Directions
Similar products from Chemical Supplies

Barium Hydroxide Octahydrate %99.0 100g

Barium Carbonate White Powder %99.0 100g

Silver Nitrate, Crystals, Chemical Lab Reagent ,10g, 99,9% Pure

HEXAMETHYLENETETRAMINE (Hexamine) ONE POUND, PYRO grade

Salicylic Acid Pure Powder 100 g Beta Hydroxy BHA 99.5%-100.5%

NAA water soluble powder ,NAA-Na 98% 200g

Lithium sulfate anhydrous, reagent, 99.0+%, 50g

Ferrous oxide / Iron (II) oxide (FeO) / CAS Number 1345-25-1 / Iron monoxide

Silica gel 100-250 / White powder / 0.1-0.250 mm/ 10 grams

Ferric Oxide / Iron (III) oxide (Fe2O3) red / CAS Number 1309-37-1 / 10 grams

Sodium Hydroxide -Lye -Caustic Soda NaOH 99.8 % Pure 8 Ounces in 2 Bottles USA

Sodium Hydroxide - Lye -Caustic Soda NaOH 99.9% Pure 5 Ounces in plastic bottle

Sodium Hydroxide -Lye -Caustic Soda NaOH 99.8 % Pure 10 Ounces in 2 Bottles USA
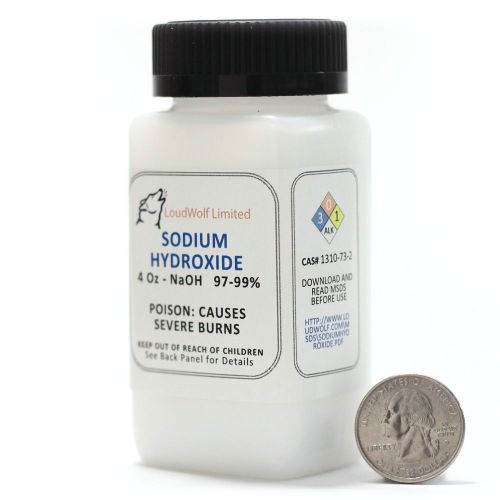
Sodium Hydroxide - Lye -Caustic Soda NaOH 99.9% Pure 4 Ounces in plastic bottle

Bee Venom Powder - Apitoxin (1 gram)

Silica Gel Desiccant Beads Orange Indicating 250ml

Cupric Chloride 30ml 98.8% Purity
People who viewed this item also vieved

GILIAN 513DRC AIR SAMPLER CHARGER B-400637

LFS-113D Pump GILIAN LOW FLOW SAMPLER

NEW Erlenmeyer Flask, 10mL, 19/22 Outer joint

2x2pcs/set 36cm Spcial Multi-line Glass Plate,#401839, for ABI 377 DNA Sequencer

SWAGELOK 1/4 x 1 SS 400-1-16 Compression x Male Pipe SS 316 NEW

160 Strips Range 1-14 pH Test Paper Urine Saliva Acid Alkaline Litmus Tester

Black Brix Fruit Juice Beer Sugar Wine Wort 0-32% Refractometer Tools Test Set

8-NUPRO METERING VALVES / 2-MATHESON VALVES #3161 - 4 CONNECTORS

Case of 360 NEW Oasis 20-dram Vials & Caps + 18 Snap Caps Pill Bottles
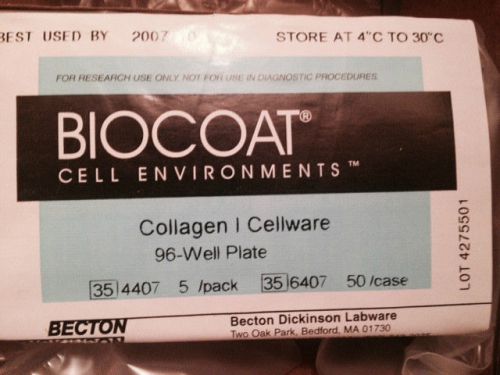
BioCoat™ 354407 COLLAGEN CELLWARE 24-WELL PLATE 5/PACK

Whatman Grade DE81 Ion Exchange Cellulose Chromatography Paper 2.5cm 100 pack
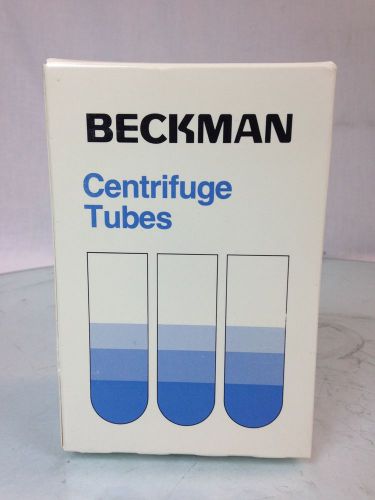
BECKMAN #331372 POLYALLOMER CENTRIFUGE TUBES 9/16" x 3 1/2" 50/PK

NIB sealed Millipak Express 40 Filter Unit 0.22um Catalogue Number : MPGP04001

Millipore Stainless Steel Pressure Filter Holder With tools

Lot of 37) Fisher Castaloy Laboratory Round Buret Flask Tube Glass Holder 2" ID

*NEW* Gilson Recorder Cable With Tinned Leads for 112 UV detector And Other Unit

Tested ProntoSIL 120-5-C18-AQ, 250 x 4.6mm, 5u HPLC column; 2546 F184PS050
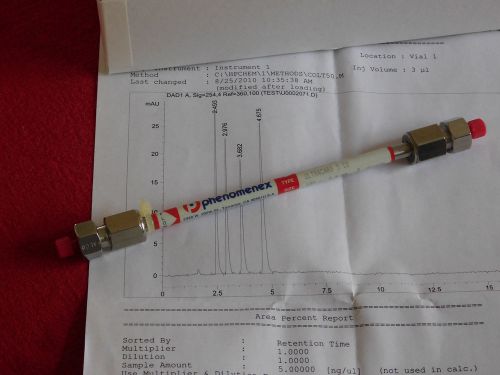
Tested Phenomenex Ultracarb 5 C8, 150 x 4.6mm, 5u HPLC column; 00F-2134-E0

2X Gilson Pipetman adjustable, P20
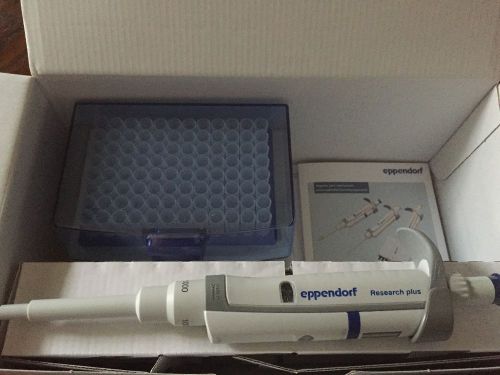
Eppendorf Research Plus Pipette (100 - 1000 microliters)

SEOH Cover Slips Plastic For Microbiology 18x18mm pk 100
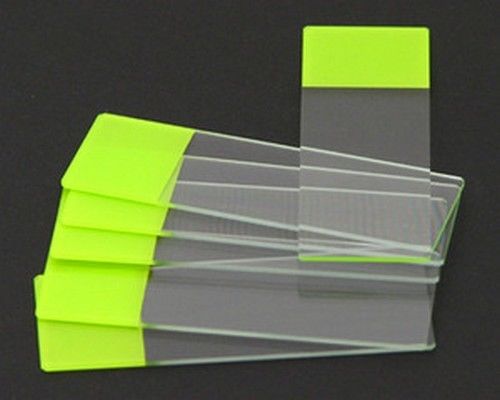
SEOH Microscope Colored End Label Microbiology Slides Green pk of 72
By clicking "Accept All Cookies", you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts.
Accept All Cookies