US $39.95
| Condition: |
New: A brand-new, unused, unopened, undamaged item in its original packaging (where packaging is
applicable). Packaging should be the same as what is found in a retail store, unless the item is handmade or was packaged by the manufacturer in non-retail packaging, such as an unprinted box or plastic bag. See the seller's listing for full details.
...
|
Brand | Contec |
| Country/Region of Manufacture | United States | ||
| Model | SONOLINE B |
Directions
Similar products from Pre-Birth Heart Monitors

Philips M2738A Avalon Leg Plate Fetal Monitor FM-20 30 40 50 Free Shipping!
Huntleigh D920 Fetal Doppler with battery (O ring glued, missing front clip)

Lot of 3 Summit Stereo Fetal Dopplers with missing front clips

Huntleigh FD1+ Fetal Doppler with FHR display (missing front transducer clip)

Newly 2.5 MHz Fetal Doppler Fetal Heart Monitor LCD Display & Gel Women Fast

Newly Selling Fetal Doppler 2MHz without LCD Display Monitors Good

Newly Fetal Doppler 2MHz without LCD Display Heart Monitors Good Fetal

USA FDA Hot Handheld Baby Heart Sound Pocket Fetal Doppler Fetal Monitor,LCD+GEL

Promotion for Fetal Doppler 2MHz with LCD Display & Rechargeable Batteries

Sonoline B 3mhzFetal heart doppler /Backlight LCD FDA and gel included USA Store

Jumper AngelSounds 100S Fetal Baby Doppler Heartbeat Prenatal Monitor Listener
2015 new Fetal Doppler 3MHz w Color LCD Back Light & Heart Beat Waveform
2015 NEW Fetal Doppler w LCD, fetal heart monitor 3MHz waterproof probe

US Seller Sonoline B fetal Doppler, Baby Heart Monitor, 3Mhz probe, Gel, FDA,New
Sonoline B Pocket Fetal Heart Doppler Backlight LCD 3mhz Probe hand-held unit
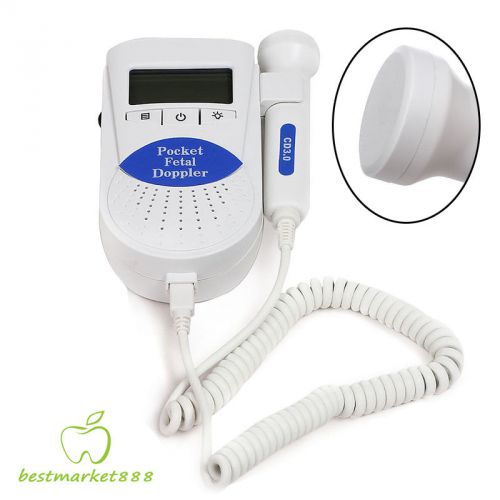
2015 NEWEST VERSION 3Mhz SONOLINE B BABY DOPPLER PRENATAL HEART MONITOR

2015 NEW Design Fetal Prenatal 3MHz Heart Rate Monitor Doppler CE

FDA Brand new 8.0 MHZ waterproof Probe Vascular Fetal Doppler Monitor 2015 new

CE&FDA Vascular Fetal Doppler Monitor with 8MHZ Vascular Probe

New arrival main unit + 8.0 MHZ Probe Vascular Doppler Monitor
People who viewed this item also vieved

Nicolet Bravo/spirit EMG/EP Amplifier

MasimoSET PC Series Patient Cable PC08

Tram RAC-4A, Solar 8000M, Dell 19" Monitor Attaches To Cart, Many Attachments
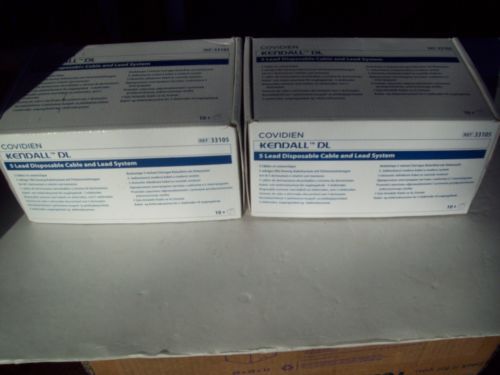
2 Boxes of 10 Sets ea Covidien Kendall DL Lead Systems REF 33105
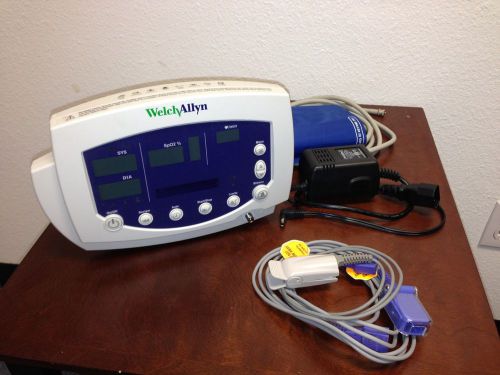
Welch Allyn Vital Signs Monitor 300 Series, 53N00

GE Medical Systems Tram 451 Module

Philips Medical M3001A Multimeasurement Server Module (MMS)

Varian 889253-02 IC Chip Software

R114600 Burdick E350 ECG EKG Machine

5 lead ECG EKG cable to patient monitor wires
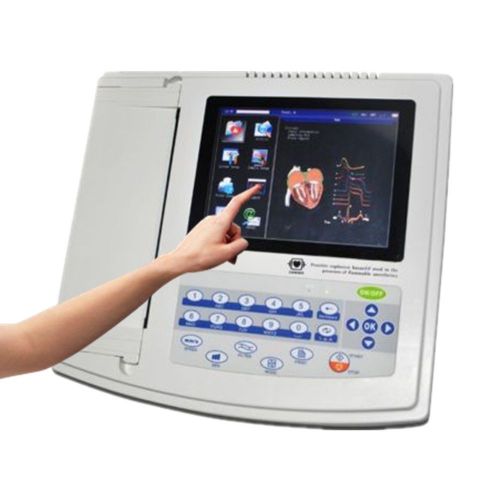
8"TFT LCD Digital Electrocardiograph ECG EKG Machine 300 Cases with ECG Software

Critikon 2604 Soft-Cuf Blood Pressure Cuff 23-33cm Long Adult

Critikon 2504 Soft-Cuf Blood Pressure Cuff 31-40cm Large Adult

Omron BP710 3 Series Upper Arm Blood Pressure Monitor Good condition

Masimo LNCS Inf SpO2 Infant pulse oximeter adhesive sensor (20 sensors)

3 Piece Lot Precision Medical Timeter Oxygen Flow Meter 15 LPM 10327

Two (2) NEW Nellcor Pulse Oximetry Cables Catalog # SCP-10

MASIMO LNCS DBI ADULT SPO2 Reuseable Sensor Ref # 2653 ***NEW***
By clicking "Accept All Cookies", you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts.
Accept All Cookies