US $7,250.00
| Condition: |
Used: An item that has been used previously. The item may have some signs of cosmetic wear, but is fully
operational and functions as intended. This item may be a floor model or store return that has been used. See the seller’s listing for full details and description of any imperfections.
...
|
Brand | Stryker |
| Country/Region of Manufacture | United States | ||
| Model | 406-800 |
Directions
Similar products from Surgical Instruments

Mizuh OSI Advanced Control Base Modular Table System Controller Remote 6807-1
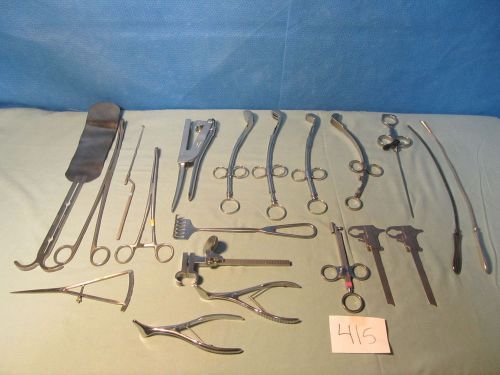
Lot of Assorted Surgical Instruments Set (QTY-20)

Lot of Assorted Surgical Instruments Set (QTY-44)

ValleyLab Force Electrosurgical Generator SSE4 W/ Rolling Cart

ZIMMER ATS Low Pressure Tourniquet Dual Port Cuff 60-7400-002 Contour Arm Cuff

Valley Lab Force 2 Electrosurgical Unit

WEM USMI SS-601MCa Electrosurgical Generator W/ Argon 4 Canady Plasma Coagulator

LAMP FOR SCIALITIC SURGICAL SKYTRON

OSI leg knee arthroscopic holder Positioner

3M 8630 DuraPrep Latex Free Iodne 26ml Alcohl Surgical Solutions Lot Of 40

Mitsubishi P40U Black / White Printer - Ultrasound - Imaging
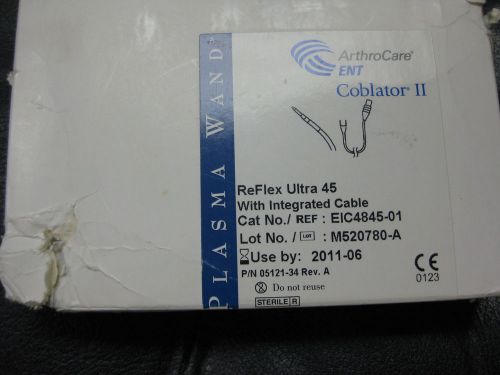
ARTHROCARE ENT COBLATOR II PLASMA WAND REFLEX 45 EIC4845-01

10 Vollrath Kidney Shaped Basins #8860

11 Vollrath Kidney Shaped Basins #88600
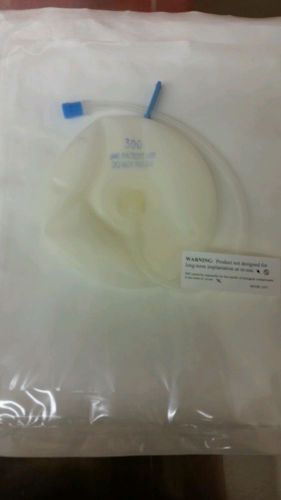
Allergan Saline Breast Implant Sizer Moderate Profile 300cc Ref SZ68300

COVIDIEN ROTICULATOR Auto suture Articulating stapler 55mm - 3.5mm #017612
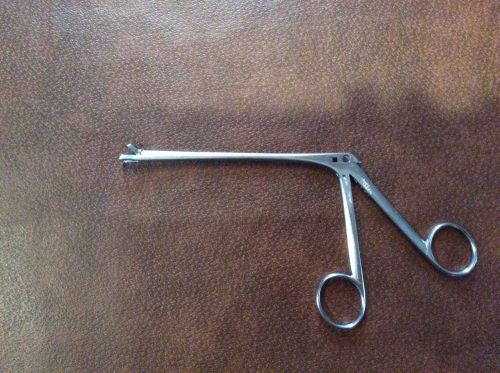
Storz N6171 Meltzer Adenoid Punch ENT Plastic Surgery Germany VET
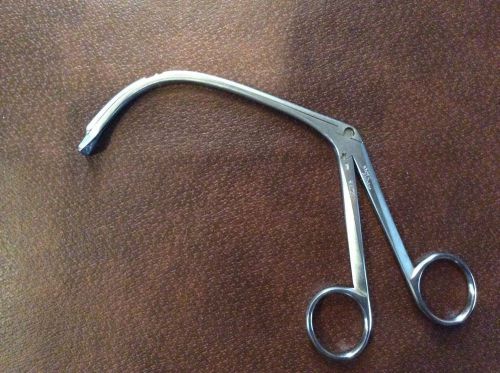
Storz N6179 Ronis Adenoid Punch Forceps ENT Plastic Surgery Germany VET

Scanlan 3788-04 Bayonet Style Micro Scissors Nuero Surgery Cardio Germany VET
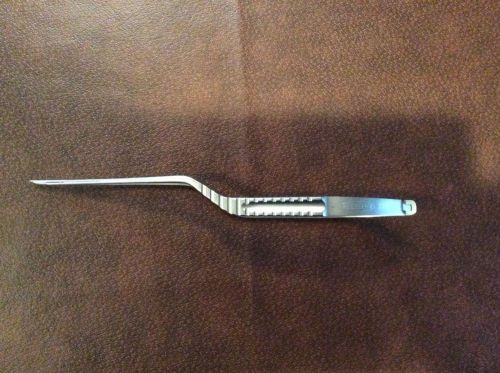
Scanlan 3788-05N Bayonet Style Micro Scissors Nuero Surgery Cardio Germany VET
People who viewed this item also vieved

T/C CNC Pin & Wire Cutter Orthopedic Surgical 8.5" Angled CUTTING CAP 3/32" MAX

STRYKER 5100-34 TPS SAGITTAL SAW - TESTED WORKS GREAT! MONEY BACK GUARANTEE!!

Orthopedic Bit For Quick Coupling Screwdriver 7", 2.5 & 3.5 mm 2 Pieces
Doctor Nurse Healthcare Medical Professional Training MORE Tracking Software CD

Medtronic Physio-Control 09-10417-0 5 Lead Electrocardiograph (ECG/EKG) Cable

NATUS BIOLOGIC SleepScan CEEGRAPH IV EEG NEURO-MONITORING SYSTEM W/ Accessories

Chattanooga Intelect Model 900 4 Channel Ultrasound Therapy Free Shipping!

Solmed Plus IR-3000 Infrared Lamp
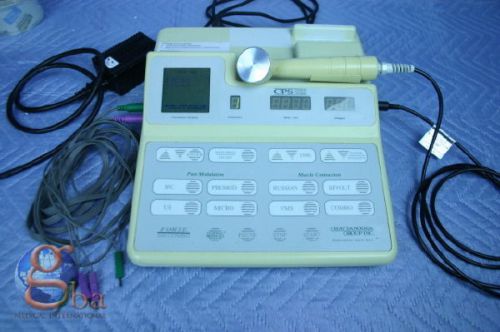
Chattanooga 200 CPS Forte 2 Channel Combination Combo Unit With Ultrasound
Nellcor Puritan Bennett 740 Ventilator

Spirometrics flowmate 2500 lte

PARI VIOS NEBULIZER 310B0000 REPLACEMENT PUMP WITH FREE SHIPPING

Castrator for Bloodless castration ( Emasculatome ) 19"

3 Canine Mouth Gag 4.50" 5.76" 6.75" SURGICAL VETERINARY INSTRUMENTS
CE Veterinary, Ultrasound Scanner Diagnostic CMS600B-3+Endorectal Linear Probe

Lloyd Galaxy Hy-Lo Chiropractor Chiropractic Electronic AdjustableTable

POSTURE PUMP CERVICAL POSTURE PUMP SPINE TRAINER Missing Chinstrap

The Spinalator Intersegmental Traction Table Chiripractic PT Physical Therapy
By clicking "Accept All Cookies", you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts.
Accept All Cookies