US $4.99
Directions
Similar products from Chemical Supplies

Nickel(II) chloride hexahydrate 99% 1000Gr

Sodium fluoroaluminate, 99% reagent 250g, CAS number: 13775-53-6

Potassium bromide 98.5%, reagent 20g, CAS 7758-02-3

Sodium fluoride 99%, reagent 20g, CAS 7681-49-4

Lithium carbonate, 99% reagent 20g, CAS 554-13-2
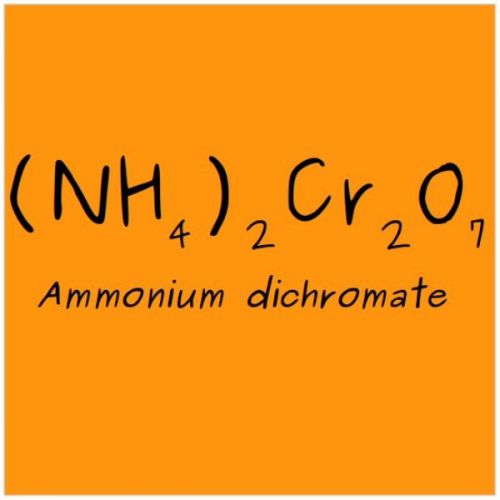
Ammonium dichromate, VESUVIAN FIRE, 99.7% reagent 20g, CAS 7789-09-5

Potassium bifluoride, pure reagent, 20g, CAS number: 7789-29-9

Zinc Oxide Powder - Non-Nano and Uncoated - Pharmaceutical Grade (99.9% pure) as

Research Product International Guanidine HCL 500g

Barium Carbonate, BaCO3, pure chemical, 16 oz

Sodium Acetate powder (HOT ICE), 16 oz

Research Products Internattional AGAROSE Optimized Grade 500G

Research Products International AGAROSE 25G

Sodium Thiosulfate, Pentahydrate (8 oz) FREE SHIPPING

99.9% High Purity TANTALUM Metal Cylinder Element Periodic Table 1.5" d x 9/16"L

PH10107B-03-B Laboratory pH Electrode

RED IRON OXIDE Fe2O3 TWO POUNDS used in THERMITE

Sodium Acetate, 4oz, Food Grade 99.9% Pure, Sturdy Bottle FROM USA

Sodium Carbonate, 4oz, Food Grade 99% Pure, Sturdy Bottle SHIPS SAME DAY
People who viewed this item also vieved

2ml TRANSFER PIPETTES , PACK of 100 POLYETHYLENE PIPETS, BIOLOGIX USA

Nalgene HDPE bottle Only (No Cap) 30mL / 1oz Bag Of 50.

HPLC column Waters XBridge C18 5 um 2.1x50 mm cat no 186003108
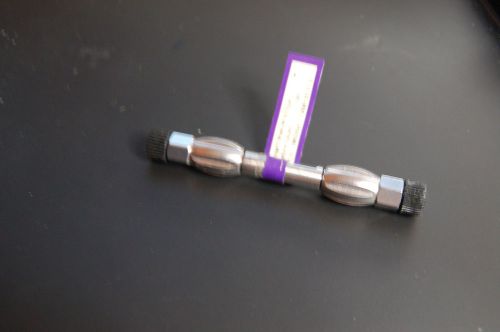
HPLC column Waters XBridge Shield RP18 C18 3.5 um 4.6x50 mm cat no 186003042

Test Tube Rack Stand Plastic for 60 Tubes x 16mm Stackable Yellow

Cole Parmer "Jiffy Jack" 3.25" X 3.5" Precision Lab Jack

Vintage Flask Screw On Lid Older Bar wear Drinks Bubbles In Glass Display

18mm snaketailed glass on glass bowl

North Safety Products Flock Lined Gloves, Size 10, 12 Pairs (NSP LA132G/10)

Anchor Brand Universal Sorbent Pad, 15" x 17", Lightweight (ANRABBPU500)

600 Kimberly-clark 36870 kleenguard A20 Breathable particle sleeve protection
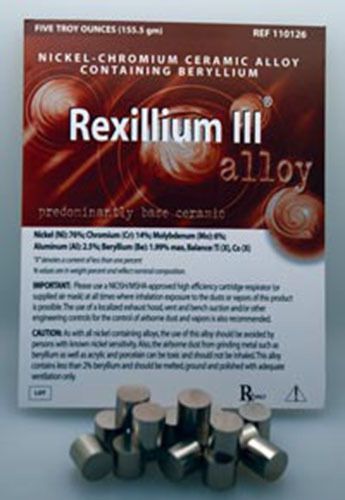
40 Ounces Of Rexillium lll For Crown & Bridges

Nalgene Biotainer Carboy w/ Handle PC 5L # 3405-16
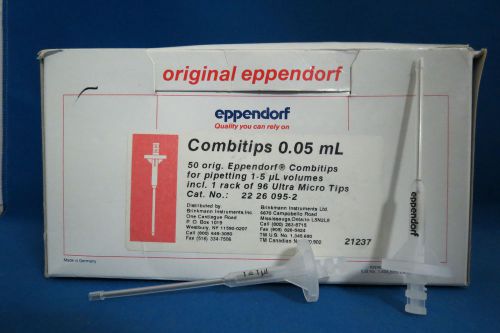
Eppendorf Combitips 0.5mL # 2226095-2 (Qty 46)

NEW Lot of 10 Furon IMP88UAM Union Male Adapter 1/2"Tube to 1/2"NPT Fittings

SMC ZSE4 Digital Pressure Switch ZSE4B-T1-26L
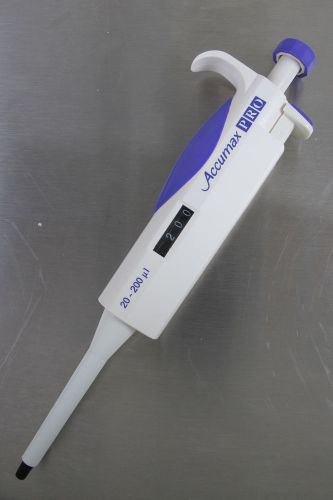
Accumax PRO Pipette Variable Volume 20 - 200 ul FVAP-800

NEW Hanna Instruments Chlorine, Free & Total High Range 100 tests HI93734-01

Waters Performance Maintenance Kit, P/N 700 001 329

BEL-ART - SCIENCEWARE F44171-0000 Microscope Slide View Pack, Histology Pkg of 9
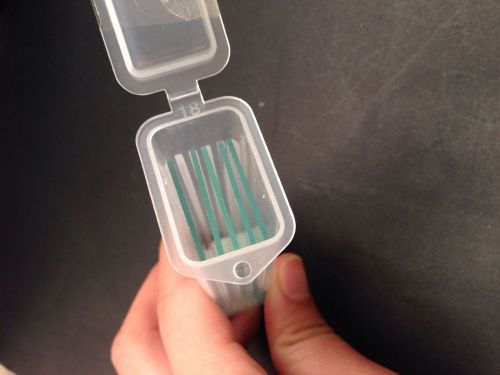
SALE! 5 PCS Reusable Laboratorial Single Concave Microscope Blank slides
By clicking "Accept All Cookies", you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts.
Accept All Cookies