US $399.00
| Condition: |
New: A brand-new, unused, unopened, undamaged item in its original packaging (where packaging is
applicable). Packaging should be the same as what is found in a retail store, unless the item is handmade or was packaged by the manufacturer in non-retail packaging, such as an unprinted box or plastic bag. See the seller's listing for full details.
...
|
Brand | Therapeutic Resources Inc. (TRI) |
| Country/Region of Manufacture | India | ||
| Model | TriMed TENS |
Directions
Similar products from General Treatment Supplies

CompuMed Automatic Pill Dispenser Machine "SINGLE KEY" FREE SHIP

CompuMed Automatic Pill Dispenser Machine "Power Supply" FREE SHIP

MedReady 1600 Automated Mediaction Dispenser Machine Replacement Gear Med530301

Candela InoLase Pneumatic Skin Flattening Laser

Mettler Sonicator Model 705 Ultrasound therapy Unit as pictured working
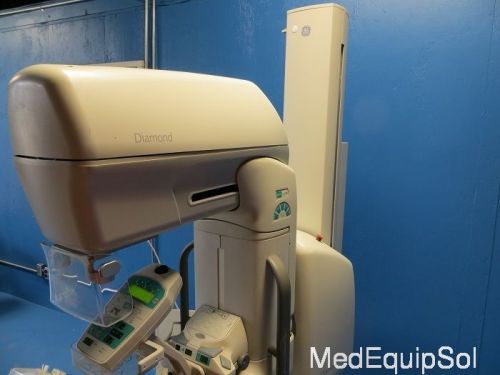
General Electric Diamond (Ref: MGX-2000) (Broken Lead Glass)

Storz Katena Accutome Pilling Weck Ophthalmic Eye Micro Surgical Instruments

Y-Tec LS-112 Fiberoptics BFW Surgical Headlight Light System 150 Watt New Bulbs

Medical America Industries Maxi Aspirator Model 603

Kendall Compression Soft Sleeves Extra Large (Ref: 5490)

Kendall Anti Embolism Stockings Medium/Regular (3 Singles)

Bair Hugger Patient Warming System

Welch Allyn Genius SureTemp Infrared TympanicThermometers (l lot)

Nikon Zoom-Photo Slit Lamp FS-3

Jay Medical Cushion Cover 15.5“ x 16“ Reference #C105 New Open Package

WELCH-ALLYN-CONMED-REF 3200-1715-ADULT ELECTRODE PAD - NEW

WELCH-ALLYN-CONMED-REF 3200-1711-PEDIATRIC ELECTRODE PAD - NEW

2007 Syneron e max Emax E-max with DSL and SR Diode Laser
People who viewed this item also vieved

Arjo MEDICAL TUB HANDICAP DISABILITY WATER THERAPY BATH Freedom Bath Swiss made

Toshiba BSM31-3064 Line Filter/American LNF/A For Nemio SSA-550A Ultrasound

Acuson CN3 Controller Plug-In Board for Siemens Sequoia 512 Ultrasound System #2

STRYKER 5100-88 TPS Micro Driver

STRYKER 4200 Cordless Driver 2

ALARIS 7130E Signature Edition Gold Single Channel IV Infusion System Pump
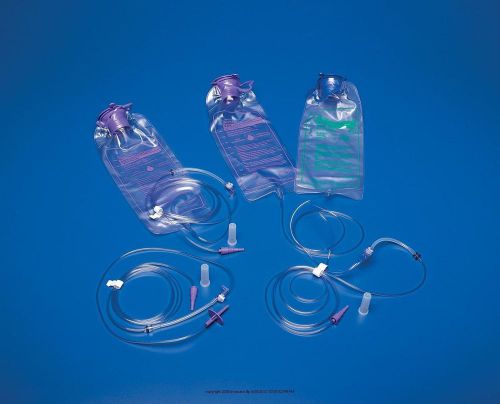
NEW KANGAROO JOEY FEEDING PUMP BAG SET 1000 ML 30 CT
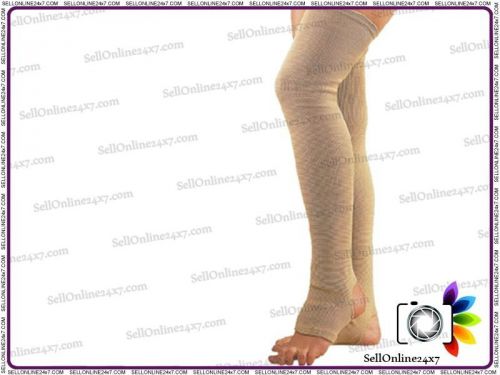
BRAND NEW VARICOSE VEIN STOCKINGS-RELIEF TO TIRED,ACHING AND HEAVY - LARGE

ADJUSTABLE SHOULDER SUPPORT DISLOCATION INJURY (3)FOR LEFT,RIGHT SHOULDER-MEDIUM

HI QUALITY UMBAR CORSET BELT -PERFECT IMMOBILIZATION TO LUMBAR SMALL SIZE
By clicking "Accept All Cookies", you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts.
Accept All Cookies