US $460
| Condition: |
New: A brand-new, unused, unopened, undamaged item in its original packaging (where packaging is
applicable). Packaging should be the same as what is found in a retail store, unless the item is handmade or was packaged by the manufacturer in non-retail packaging, such as an unprinted box or plastic bag. See the seller's listing for full details.
...
|
Brand | Contec |
| Country/Region of Manufacture | China | ||
| Model | CMS8000 | ||
| Country of Manufacture | China | ||
| MPN | Does not apply | ||
| UPC | Does not apply |
Directions
Similar products from Other Monitoring Devices

419723-001 ASSY DAS DASH 3000 MODULE @ (122611)
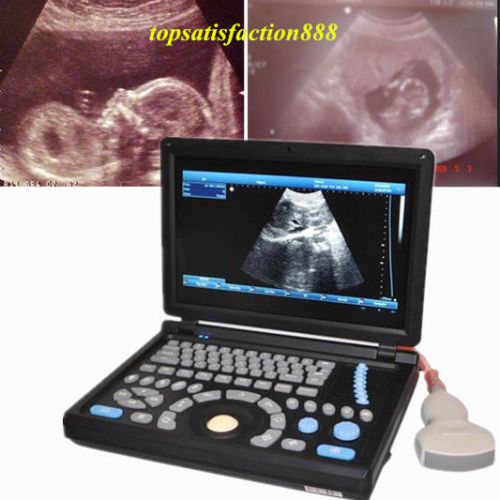
3D PC Digital Laptop 3.5Mhz abdominal Probe +Ultrasound ScannerMachine Main Unit
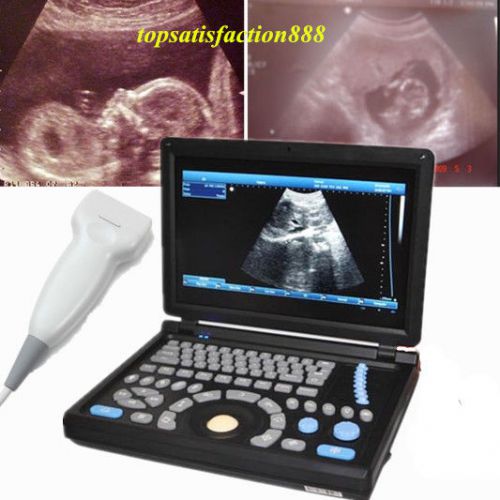
3D PC Digital Ultrasound ScannerMachine Laptop Main Unit+7.5Mhz Superficia Probe

PHILIPS IntelliVue Module Rack M8048A w/ modules UNTESTED / Cables
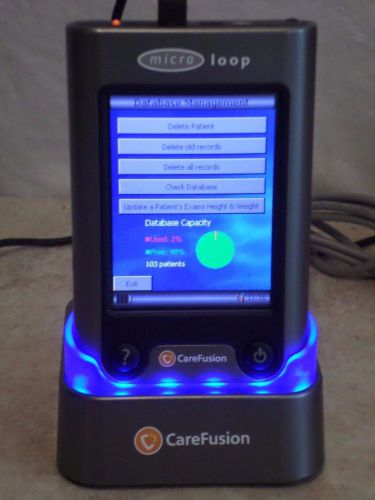
Carefusion Micro Loop Microloop UK Spirometer Spirometry Portable

High Quality Masimo LNOP DCI Compatible Finger Probe SPO2 Sensor #030
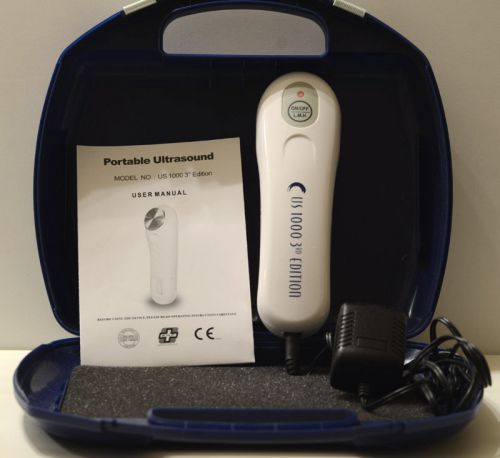
US Pro 1000 3rd Edition Portable Ultrasound Therapy Unit

NIBP HOSE CRITIKON DINAMAP 8100 8100T 9300XL 1846

GE marquette Tram 450sl multiparameter module 450 sl - Good Working Condition

Philips M8040-60101 MMS Mounting Kit M3001A M3015A

Welch Allyn PROPAQ 100 Series And Power Supply Cord

Heritage Medcall Sentury Station

Curbell Gen111 Pillow Speaker 3408-001

Caradyne Criterion 40 Monitor VG+
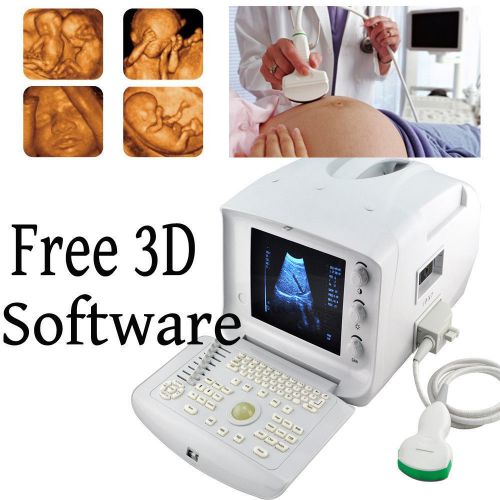
3D NEW Portable Ultrasound Scanner machine system CONVEX+ FREE 3D WORKSTATION

3D PC Color Full Digital Laptop Ultrasound Scanner Linear CE FDA Veterinary VET

Build-in 3D PC 10.4"TFT LCD Color Full Digital Laptop Ultrasound Scanner Linear

Marquette Eagle Medical/Hospital Patient Monitor/Display NELLCOR SENSORS ONLY

FUKUDA DENSHI LW-5560N CENTRAL TELEMETRY RECEIVER @ (117310)

MONITOR LINE ISOLATION 50UA 17W 115VAC
People who viewed this item also vieved

Critikon Soft-Cuf Neonate #4 Double Tube 7-13cm Ref:2524

CONTEC 24 hours Ambulatory Blood Pressure Monitor,CONTEC06+free software+3 cuffs

GE DATEX OHMEDA AS/3 AS5 M-NSAT nellcore

BLOOD PRESSURE ARM CUFFS MISCELLANEOUS BRANDS MIXED LOT OF 5 (ITEM #1725 A/ 3)

Covidien Kendall DL Disposable Cable and Lead Wire System 33135 - NEW

Brand New 10 Lead ECG/EKG Cable with Leadwire for GE Marquette

Health Care Portable Handheld home ECG EKG Heart Monitor Gift

DATEX OHMEDA OXY-W-DB OXY TIP + PLUS WRAP SENSOR - NEW
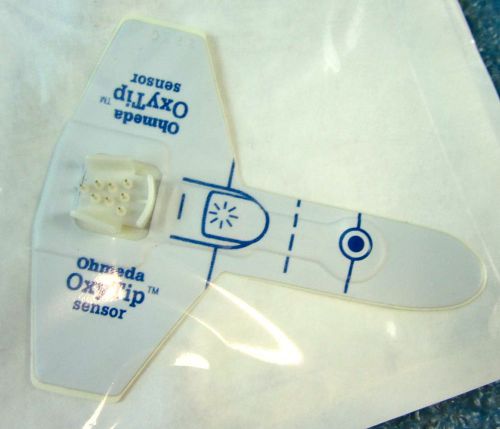
*17pc/LOT* OHMEDA 6038-6000-006 OXY TIP SENSOR, ADULT ADHESIVE OXYGEN SENSOR -

DATEX OHMEDA SAS-W SAS-W..00. FLEXSTAT SENSOR, PULSE OXIMETRY SpO2 SENSOR - NEW

SpaceLabs Patient Monitor, (Model 90308-15)

HP Hewlett Packard SpO2/Pleth Module M1020A

Datascope Accutorr 3, 4 Series Patient Monitor

Hewlett Packard M1276A Module Rack

New 1 X Baby Doppler Heartbeat Prenatal Monitor Angel Sounds Pink FDA CE Proved
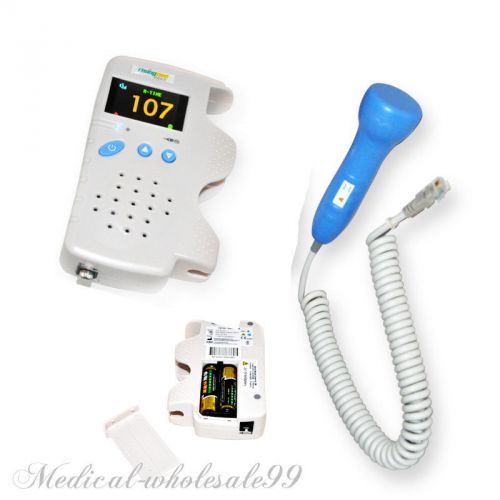
2014 new Fetal Doppler 3MHz LCD Back Light & Baby Heart Beat Waveform ce
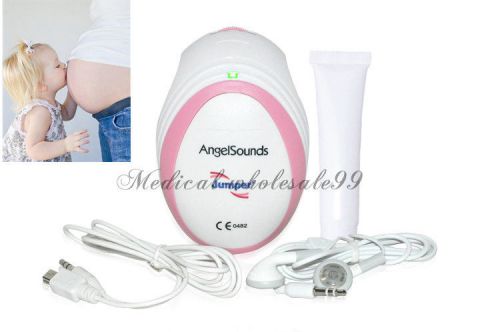
BID** HOT! Baby Angels heart sound fetal doppler Prenatal Monitor Angelsounds

2014 New Fetal Doppler 2.5 baby prenatal Heart Beat Monitor w LCD display
By clicking "Accept All Cookies", you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts.
Accept All Cookies