US $370
| Condition: |
New: A brand-new, unused, unopened, undamaged item in its original packaging (where packaging is
applicable). Packaging should be the same as what is found in a retail store, unless the item is handmade or was packaged by the manufacturer in non-retail packaging, such as an unprinted box or plastic bag. See the seller's listing for full details.
...
|
Brand | CONTEC |
| MPN | Does not apply | ||
| Model | PM60A | ||
| UPC | Does not apply |
Directions
Similar products from Pulse Oxymeters

US seller, Finger Tip Pulse Oximeter Blood Oxygen SpO2 Monitor ,FDA CE Approved

CONTEC USA! Finger Tip Pulse Oximeter Blood Oxygen SpO2 PR Monitor CMS50DL Black

Schiller Sp-ox Handheld Pulse Oximeter
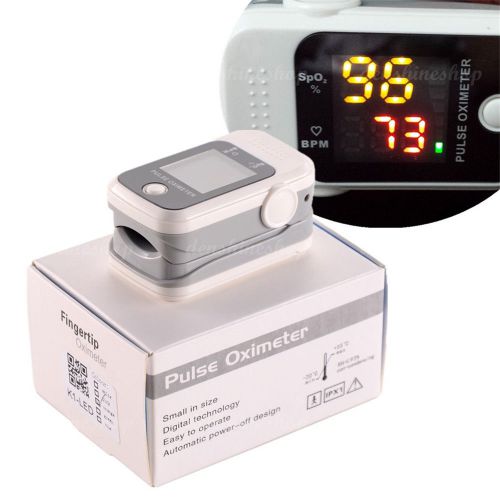
FDA CEFinger Pulse Oximeter oximetro Blood Oxygen SpO2 PR Monitor

Color Finger Tip Pulse Oximeter Blood Oxygen SpO2 PR Monitor LED FDA CE

CE finger pulse oximeter LED Waveform SPO2 PR monitor blood oxygen FDA SALE

FDA CE Finger Pulse Oximeter Blood Oxygen Saturation SPO2 PR Color LCD monitor

BID CE Finger Pulse Oximeter Blood Oxygen Saturation SPO2 PR Color LCD Blue
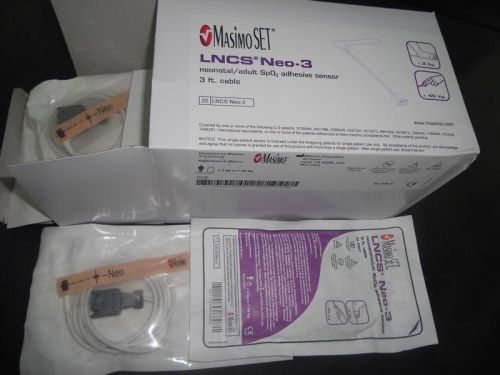
Masimo LNCS NEO-3 SENSORS ref 2320 NEW!

Handheld Veterinary Pulse Oximeter with Tongue SpO2 Probe+PC Softwar Vet/Animal
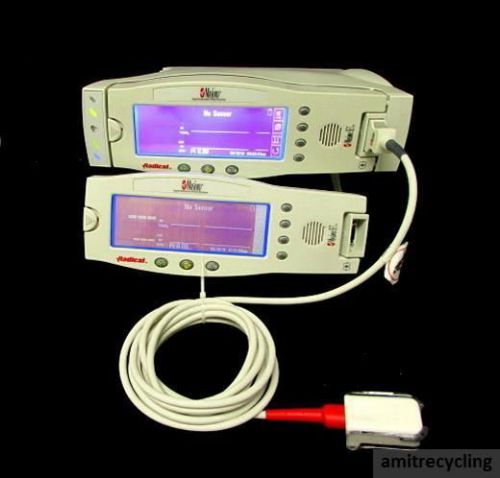
Lot Masimo Radical Handheld Pulse Oximeter SpO2 V4 w/ finger probe extension

Brand New Masimo Radical 7 Rainbow Touch Screen + SpO2 Sensor

Nonin 3231 USB Pulse Oximeter New in Box !!

Masimo LNCS NeoPt Neonatal SpO2 sensors (20 sensors)

CMS 50-DL Pulse Oximeter with Neck/Wrist cord

CVS Pulse Oximeter Model C20 Includes Case

Nonin Onyx II 9550 with Mark Justice case

GE TruSignal SpO2 Sensor TS-F-D 3ft With Interconnect Cable TS-N3 10ft
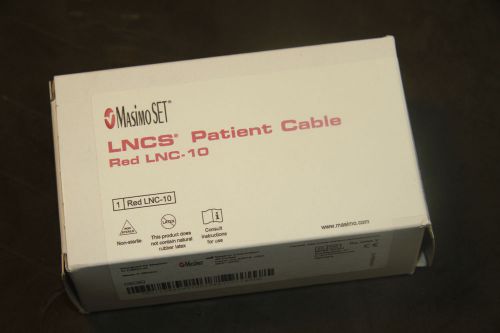
Masimo Set LNCS Patient Cable Red LNC-10-GE
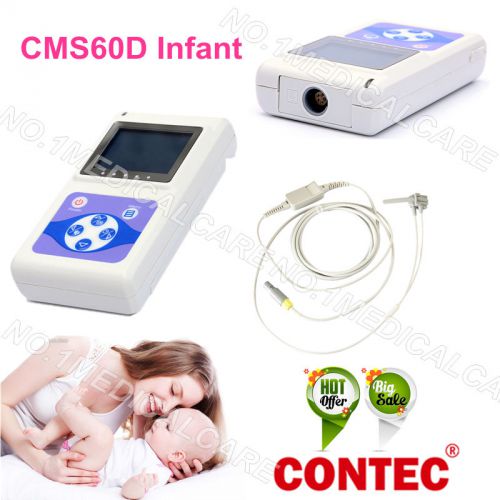
Neonatal Infant pediatric New Born Baby Pulse Oximeter Spo2 Monitor USB software
People who viewed this item also vieved
Lightly Used CK Wrist Blood Pressure Monitor Blood Pressure Monitor home English
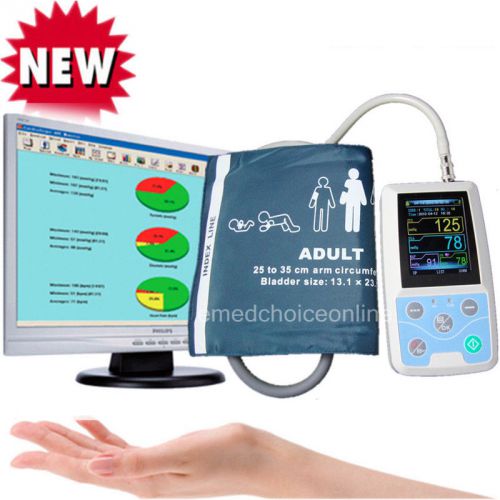
HOT Ambulatory 24-HOUR Blood Pressure ABPM +3 cuffs Holter NIBP MAPA Monitor

New Sale Reusable Thigh Double-tube Blood Pressure Cuff 46-66cm arm CM1205

for sale Large Adult Double-tube Blood Pressure Cuff 33-47cm arm CM1204
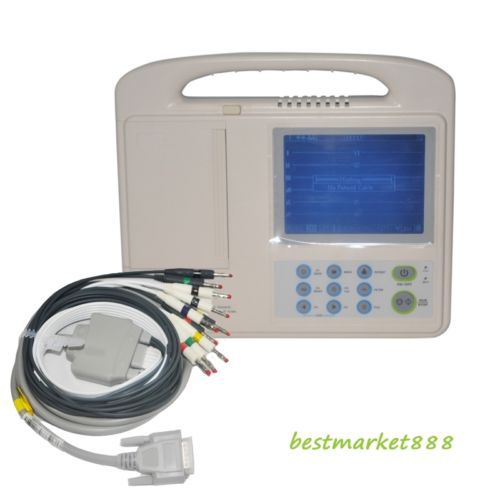
Portable Digital 6-channel 12-lead Electrocardiograph ECG /EKG Machine 40 Case

3 Channel 7 inch Color LCD Digital Electrocardiograph ECG Machine EKG Machine CE

new ECG investigation Holter Simulator GENERATOR 12 leads 1.5v alkaline battery

2015 ECG investigation Holter Simulator GENERATOR 12 leads 1.5v alkaline battery

GE Case P2 Series Medical Patient Heart Cardiac Stress Exercise Test System

Boehringer 7701 Suction Regulator 0-200 mm Hg

NEW MERCOID DA-31-153-2 PRESSURE CONTROL 120/240V-AC 2/4A AMP SWITCH

NEW Philips IntelliVue MSL Patient Monitoring Link Cable M3081-61626

HP Philips Series 50XM Fetal Monitor Ohmeda Ultrasound Toco -Parts/Repair-
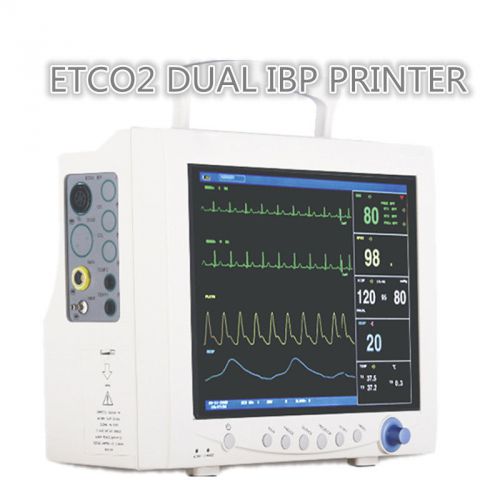
ICU PATIENT MONITOR TOUCH SCREEN ECG NIBP SPO2 TEMP RESP PR ETCO2 DUAL IBP PRINT

Prenatal heart monitor. Gift set
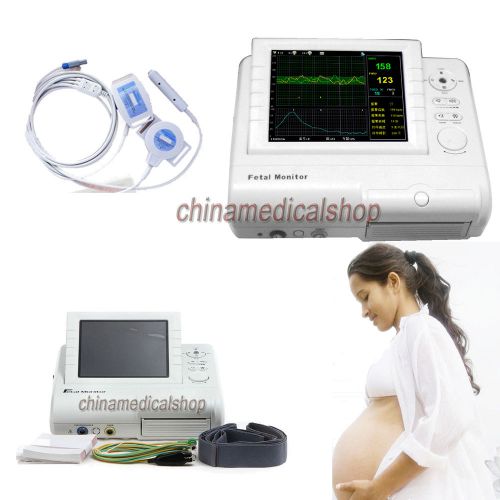
*CE* 24h monitoring Maternal&Fetal Monitor,FHR wave,Fetal move,TOCO,with Printer
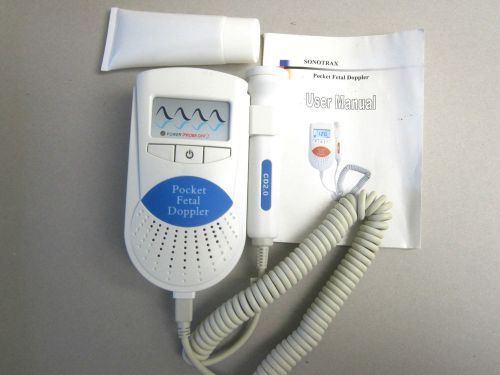
Ultrasound Pocket Fetal Doppler Heart Rate Monitor (Sonotrax-A)
CE CONTEC Brand CMS800G Fetal Monitor FHR TOCO Fetal Movement + twins probe
By clicking "Accept All Cookies", you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts.
Accept All Cookies