US $1700
Directions
Similar products from Sterilizing Equipment & Autoclave Machines

Getinge 46 Series Washer Disinfector Model 46-4-303 steris amsco lab sterilizer
Pelton and crane delta q8 220 volt sterilizer autoclave

Tuttnauer 1730M ValueKlave With Extras

AMSCO Eagle Ten Autoclave Sterilizer

Scican STATIM 1102 Steam Cassette Autoclave Sterilizer Instrument

Steris Amsco Sterilizer Printer Recorder Paper for Eagle and Century, 3 Rolls

Bioclave Laboratory 16L Sterilizer Refurbished Autoclave 110V 4 Lab + Warranty

Condenser Waste Bottle Kit for SciCan Statims (Part SCK016), OEM Part 01-100812S

Ritter M7 Speed Clave Autoclave Instrument Sterilizer with Warranty

Scican Statim 2000 Dental Sterlizer (1 year parts warranty!)

Tuttnauer Brinkmann 2340M Autoclave / Sterilizer

Premier Model B Glass Bead Dental Sterilizer for Dental & Medical Instruments
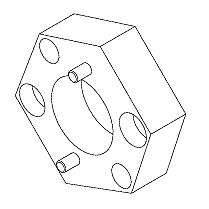
SciCan Statim 5000 SPANNER NUT - RPI# RPT364

Flinn Scientific Goggle Sanitizer SE1000 115V 25 watts cycle 60 phase 1 amp .21

Amsco American Sterilizer Company Parts P 43058-91 ????? #967

18L 304 stainless Electric and Fire Heating Portable tattoo Autoclave

Used Ritter M9 UltraClave Autoclave Sterilizer

stainless steel Dental Autoclave Steam Sterilizer Pressure Sterilization 18L

BRAND NEW! Scican Statim 2000/5000 Reservoir Cap & Filter OEM# 01-101783S
People who viewed this item also vieved

Netgear Print Server PS111W with Netgear Wireless PCMCIA card PS401

LT-05C Dual Band&Power 35W/60W Ultrasonic cleaner cleaning machine 220V

GENEVAC HT-12 HT12 ATLAS EVAPORATOR COOL HEAT TECHNOLOGY VAPOR CONDENSER VC3000D

AJ111891 Labnet Vortemp 56 Microplate Shaking Incubator S2056-A

100mm x 10mm lens or mirror optical table kinematic holder Melles Griot

MEIJI TECHNO MA551 Meiji Techno MA551 4" Chromed Pillar Extension NICE #64

LEICA GZ4 Stereo Zoom Microscope, 15X WF, 45X Max Magnification, NICE IMAGES #99

ROTARY SENIOR MICROTOME Best Supper LABGO

KINEMATICA PT-DA 30/2T-A BATCH DISPERSER AGGREGATE

NEW - Newport 860A Motorizer - Motorized Linear Actuator

Acrylic Boxes/Dessicators w/lids Set

Hammond Power Supply Power supply Model 94567

Cole Parmer Masterflex Peristaltic Pump Drive, Model 7521-50, 1-100 RPM

Gould 23-5215-11 Fanfold Chart Paper 2 Packs of New Unopened Chart Paper Thermal

Qiagen Retsch MM300 TissueLyser

WARING COMMERCIAL LABORATORY BLENDER BLENDOR HOMOGENIZER 700 MODEL 33BL479
By clicking "Accept All Cookies", you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts.
Accept All Cookies