US $145.00
| Condition: |
Used: An item that has been used previously. The item may have some signs of cosmetic wear, but is fully
operational and functions as intended. This item may be a floor model or store return that has been used. See the seller’s listing for full details and description of any imperfections.
...
|
Brand | Prentice Hall |
| Country/Region of Manufacture | United States | ||
| Model | FMO US Patent 3,080,662 |
Directions
Similar products from Educational Materials

Anatomical Foot Models- flat arch

Skeletal Hand Model- right & left
Anatomical Skeletal Hand Models-
Skeletal Hand Model- right shoulder with stand
Complete antique vintage Somso Marcus Sommer plastic Model hum eye in orbit
Genuine antique vintage Somso Marcus Sommer plastic Model of Human body
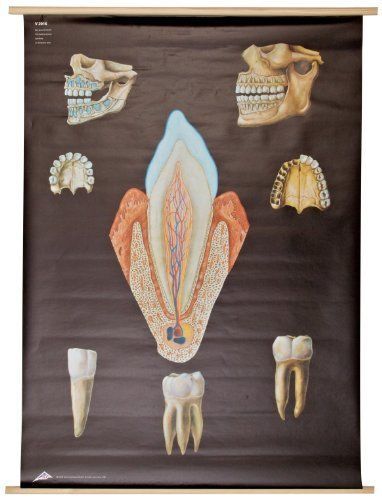
3B Scientific V2016U Healthy Denture Anatomical Chart without Wooden Rods Over
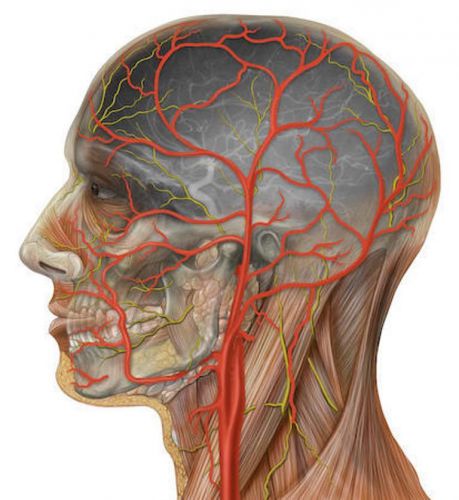
Human Head: Life size model of human head mounted on base

edu Science Frog Dissection Model Anatomy Kit for Kids

Vintage Denoyer Geppert - Bladder Section Piece Anatomical Model for torso model
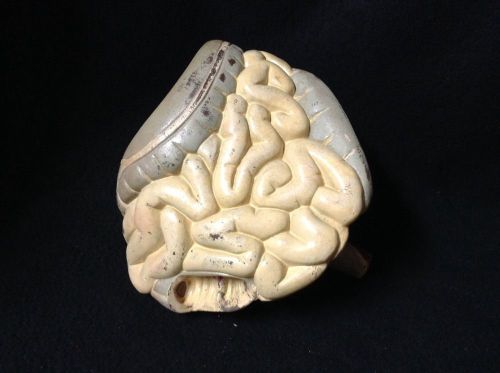
Antique - Small & Large Intestine Colon Digestive System Anatomical Model

Breast Exam - Concern Transillumination Electronic BSE Anatomical Model

Antique / Vintage Stomach Digestive System Anatomical Model Life Size
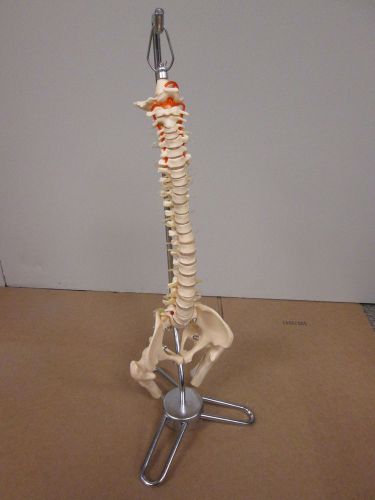
Flexible Desk-size Vertebral Column Item #: CS85X Good Condition (D-5)
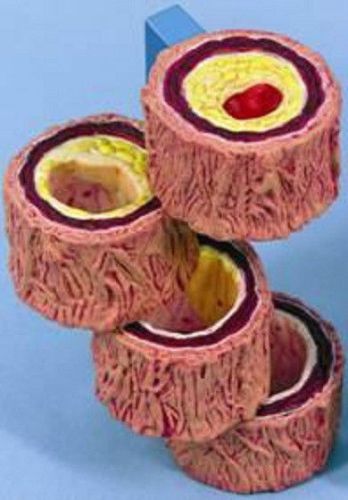
NEW Anatomical Artery Cholesterol Atherosclerosis Model

VENI-DOT Phlebotomy Nursing Training Aid Blood Drawing Phlebotomist by Elektro

Human Skull Anatomical Anatomy Skeleton Medical Model & Colored Bones LifeSize

Mr. Thrifty Skeleton Anatomy Model

ACU-ARC RULER FOR D,C, TO MEASURE CERVICAL CURVES & SPINE CURVES (NEW)
People who viewed this item also vieved
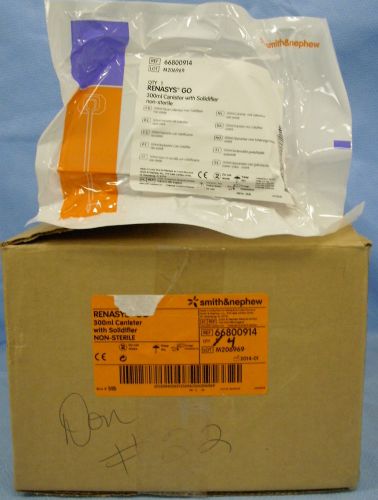
4 Smith & Nephew Renasys Go-300mL Canister w/ Solidifier #66800914

1 Box of 100 Smiths Medical Jelco Hypo Needle-Pro Devices #4287

1 Box of 24 Pouches Natus Neurology Disposable Electrodes #019-415200

1 Case/10 boxes-100ea Dynarex Electrode Skin Prep Pads #1508

CARRINGTON MOISTURE BARRIER CREAM SKIN PROTECTANT NDC53329-076-81 #CRR10401
6' VERTICL CLOSURE STRIP 221812

Dynarex Sensi Wrap Self Adherent Bandages, Tan, 3" x 5 yds, 24/CS, 3173

Dynarex Stretch Gauze Bandage Roll, 3", 96/CS, 3103
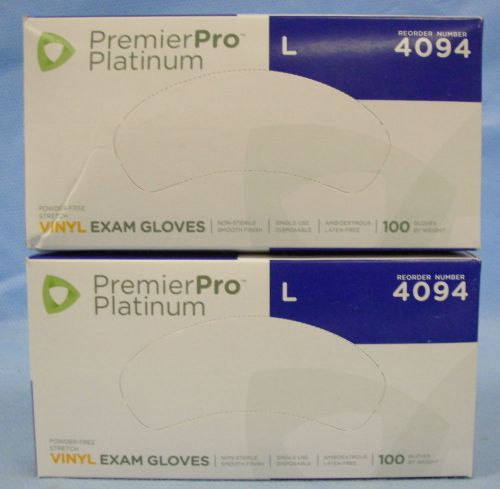
2 Boxes/100ea s2s Global PremierPro Platinum Vinyl Exam Gloves-Large- #4094
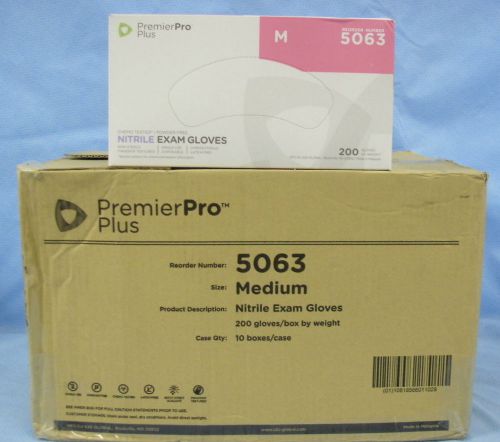
1 Case/10 Boxes s2s Global PremierPro Plus Nitrile Exam Gloves-Medium- #5063
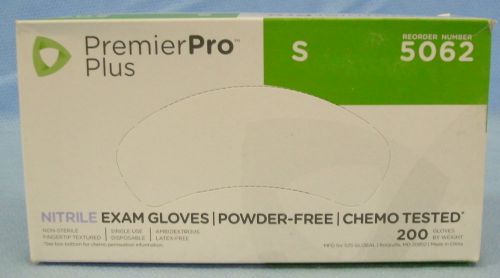
1 Box/200- s2s Global PremierPro Plus Nitrile Exam Gloves- Small- #5062
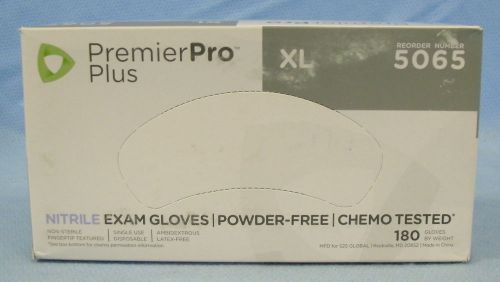
1 Box/180- s2s Global PremierPro Plus Nitrile Exam Gloves-X-Large- #5065

NEW--Kendall Monoject Oral Medication Syringes Tuberculin Syringe 1 mL(1 cc)
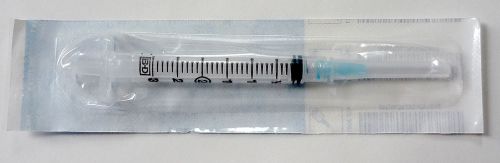
Single BD 3 ml Syringe With Luer-Lok Tip with BD PrecisionGlide needle
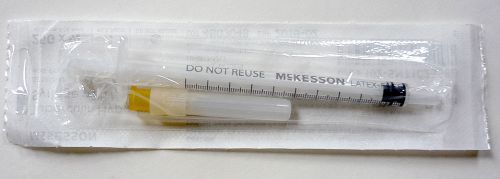
Single McKesson Tuberculin Hypodermic Syringe With Detached Needle 1cc

60ml 60cc Syringe Luer Lock Medint Box of 30 Syringes
By clicking "Accept All Cookies", you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts.
Accept All Cookies