US $199
Directions
Similar products from Pre-Birth Heart Monitors

Corometrics Model 126 Fetal Monitor *Certified*

Corometrics Model 128 Fetal Monitor *Certified*
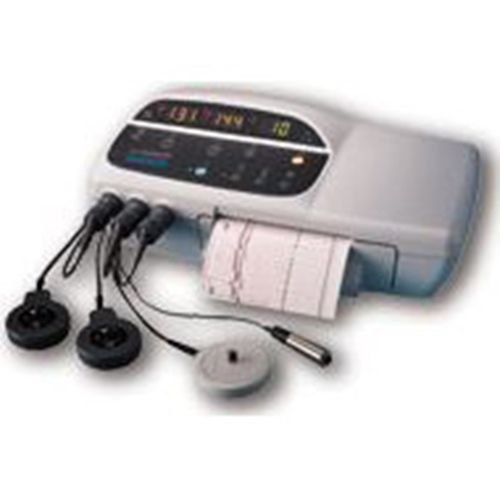
Corometrics 174 Fetal Monitor (Twins) *Certified*

Corometrics 150 (Twins) Fetal Monitor *Certified*

Wallach Fetal2EMR Thermo Paper (3 Pack)
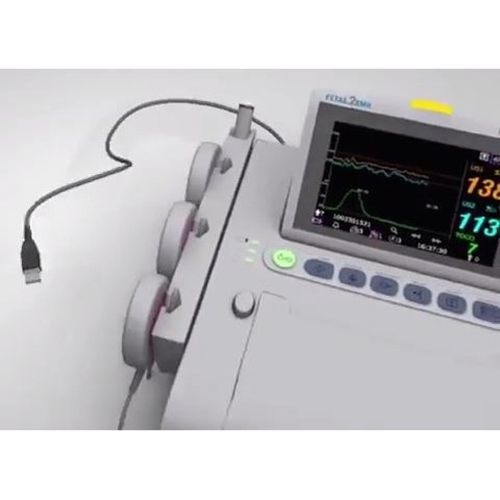
Wallach Fetal2EMR Insight Connection Cable
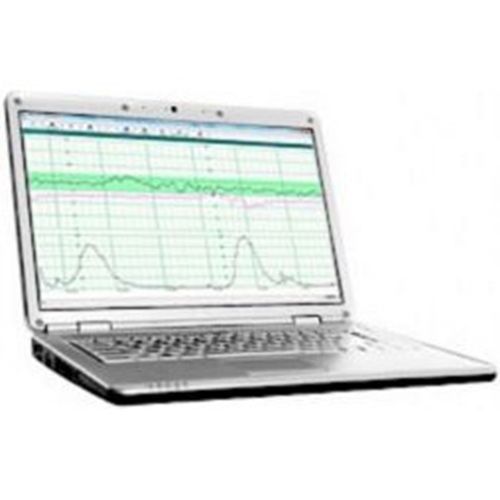
Wallach Fetal2EMR Insight Software

Wallach Fetal2EMR Event Marker

Wallach Fetal2EMR FHR Probe/Ultrasound Transducer
Wallach Fetal2EMR Ethernet Cable

Summit Doppler 8MHz Sterilizable Vascular Probe
Summit Doppler 8 MHz Vascular Probe
Summit Doppler 5MHz Vascular Probe
Summit Doppler 4MHz Vascular Probe

Summit Doppler 7.5cm Metatarsus Blood Pressure Cuff
Summit Doppler 2 MHz Waterproof Obstetrical Probe

BISTOS HI-BEBE PORTABLE HANDHELD FETAL DOPPLER HEART RATE MONITOR BT-200

Summit Doppler Stereo Headphone
People who viewed this item also vieved

Welch Allyn 5085-11 Large Nylon Zipper Case

LABTRON HOME BLOOD PRESSURE KIT WITH SPHYGMOMANOMETER AND VINYL POUCH

New Spacelabs TruLink Hose Assembly Quick Connect 94830 9' 714-0066-00

Spacelabs Non-Invasive Blood Pressure Cuff Reuseable Lot

3 lead ECG leadwire, Snap,Holter Recorder ECG Patient Cable-Apply to many models

New 14 pins TPU Nellcor Compatible SpO2 sensor Adapter Extension 3M Cable DOC-10

Burdick 10-Lead Shielded EKG Cable ,AHA, Din3.0 ,15pins, K1102N

Kenz 16 male pins / Cardioline PC-104 EKG Cable, Din3.0 / AHA, K1107N

Black Fingertip pulse Oximeter Finger Pulse Blood Oxygen SpO2 Monitor FDA Certif

Pulse Oximeter Finger Pulse Blood Oxygen SpO2 Monitor COPD Emphysema Pulmonary

Health Blood Oxygen Monitor SPO2 PR Contec CMS 50DL CE&FDA Bag/Case Warranty

Home Care Blood Oxygen Monitor SPO2 PR Contec CMS 50DL CE&FDA Free case Warranty
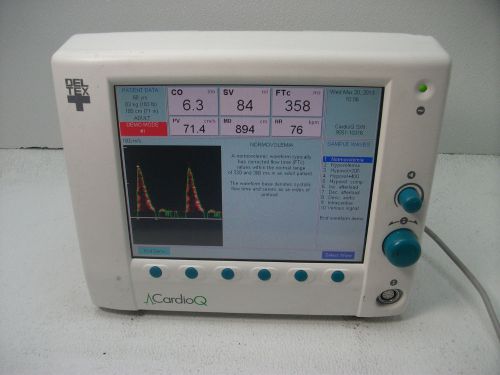
Deltex CardioQ Doppler 9051-6905

Spacelabs Digital Telemetry ECG Transmitter 90347

Spacelabs Digital Telemetry Transmitter 90343

694863 HOPKINS ISO-VITAL SIGNS KIT - ADULT
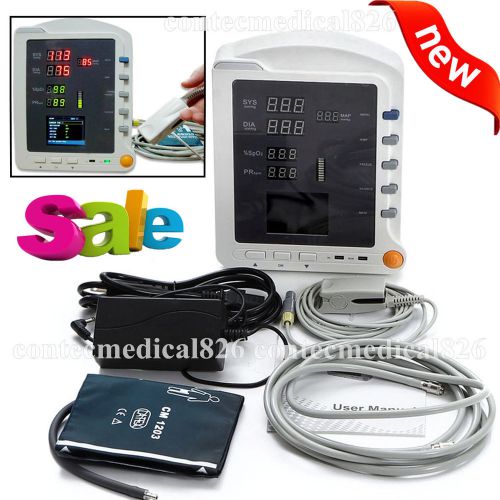
CE,FDA, New vital signs ICU/CCU patient monitor 3 parameters NIBP+SPO2+Pr

Aspect BIS Bispectral Index Monitor system Bisx EEG A-2000

Aspect Medical Systems BIS VISTA Bispectral Index Monitor system Bisx EEG

10 Pack of PROTOCOL ADULT/PEDS MAINSTREAM CO2 AIRWAY ADAPTER TRAINER 008-0367-00
By clicking "Accept All Cookies", you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts.
Accept All Cookies