US $1300
| Condition: |
New: A brand-new, unused, unopened, undamaged item in its original packaging (where packaging is
applicable). Packaging should be the same as what is found in a retail store, unless the item is handmade or was packaged by the manufacturer in non-retail packaging, such as an unprinted box or plastic bag. See the seller's listing for full details.
...
|
Brand | Contec |
| Model | CMS8000 |
Directions
Similar products from Health Condition Monitors
NEW ECTO2+ Printer 15'' colour Patient Monitor ECG NIBP PR Spo2 Temp Resp CONTEC
CE Certified CONTEC CMS800G fetal monitor,twins probe+Printer,fetal heart rate
CE Certified CONTEC CMS800G fetal monitor,FHR TOCO Fetal movement with printer
12.1''ICU Patient Monitor Multi parameters ECG NIBP Spo2 Temp Resp Etco2,CMS8000
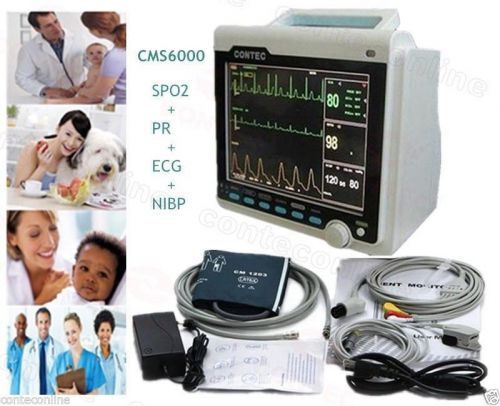
CMS6000A Patient Monitor 4 parameters NIBP SPO2 ECG/EKG Vital Signs Monitor

CE, Colour LCD Big Touch-Screen ICU CCU Vital Signs Patient Monitor,in Popular.
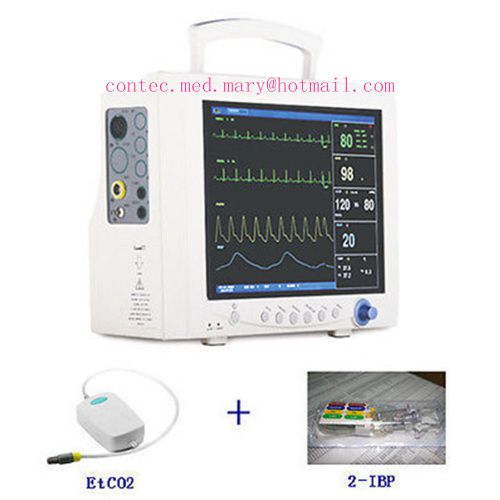
CE&FDA CMS7000 Multi-Parameter Patient Monitor EtCO2+IBP+NIBP+SPO2+PR+RESP+TEMP
NEW,with ETCO2 ,CE FDA ,Multi Parameters ICU Patient Monitor CMS7000+printer
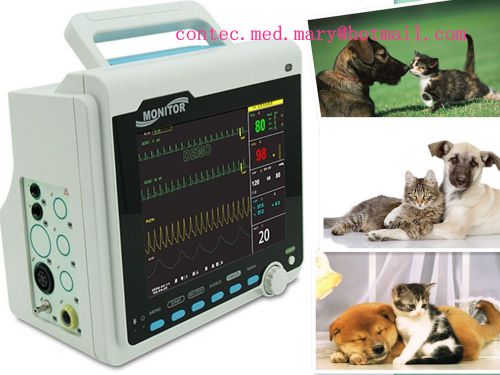
Veterinary,VET UseICU CCU Vital Signs Patient Monitor ECG NIBP SPO2 RESP TEMP PR

Hot,LEDS display patient monitor NIBP,SPO2 ,pulse rate Vital Signs ICU Monitor
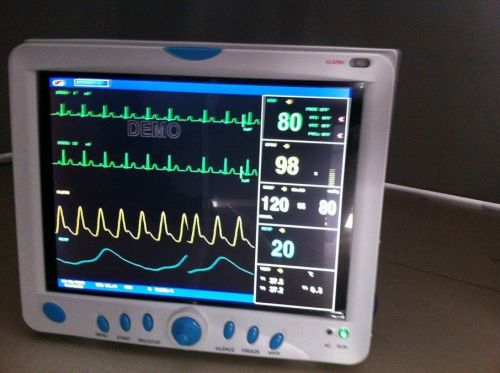
CE FDA ,With ET-CO2 ETCO2 CONTEC CMS9000 Muitl Parameters, ICU Patient Monitor

Tina TCM4 Series Blood Gas Monitor Patient Monitor

Abbott Critical Care Systems 3300 Cardiac Output Computer Cardiac Output Monitor

Nellcor N-1000 Multi-Function Patient Monitor Display - GREAT!

GE Critikon Dinamap Pro 200 Vital Signs Monitor Patient Monitor

Welch Allyn 420 Series Spot Vital Signs Monitor
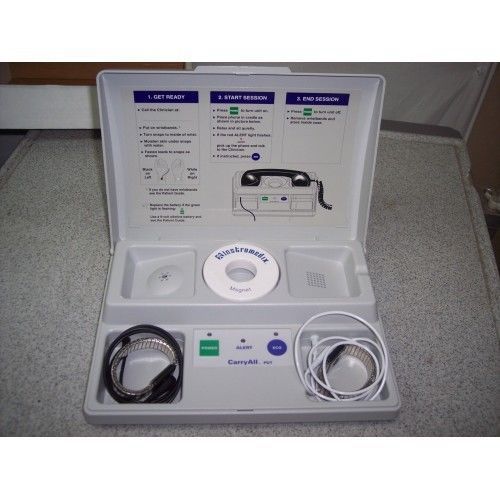
Instromedix CarryAll PDT Transmitter For Pacemaker

DATASCOPE ACCUTORR PLUS 0998-00-0444-93A PATIENT MONITOR !

BCI 6100 Model 58200A1 Medical Patient Vital Signs Monitor w/Built in printer
People who viewed this item also vieved

Sony LMD 1950MD/HD Monitor with AC 2450 MD AC Adaptor

Power Cord for Respironics REMstar Plus, Pro, and Auto Machines
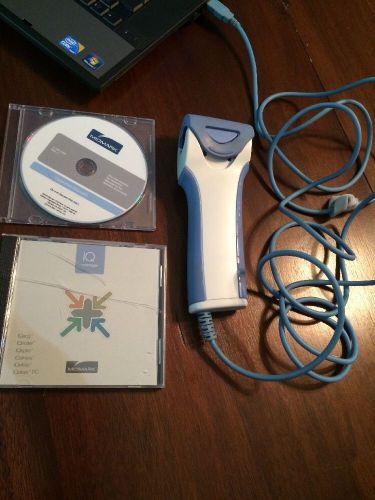
Midmark IQspiro Digital Spirometer
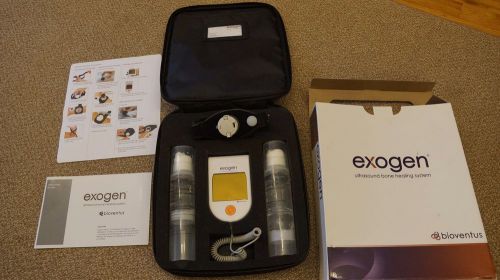
Exogen 4000 Ultrasound Bone Stimulator Bioventus 2013
LCD Sonoline B Fetal doppler Baby Prenatal Heart Monitor 3mhz +Gel *USA SELL
Summit Doppler EchoHeart 5MHz Transvaginal Obstetrical Probe

Summit Doppler Hand-Held Doppler Carrying Case
Summit Doppler L150 Doppler Package
Welch Allyn Blood Pressure Cuff Reusable Small Adult 10 *FREE SHIPPING*

Welch Allyn Small Adult 10 Durable BP Cuff With tube and connector # 008-0627-00

Welch Allyn Blood Pressure Hose - Spot Monitor LXi / Connex Series - 4500-30 5ft

Omron HEM-7120 Upper Arm BP Monitor Automatic- Fast FREE SHIPPING

Masimo Rad 8 Pulse Oximeter and Patient Cable (No Sensor)

Masimo SpO2 Cable Compatible MASIM LNCS

Athlete Finger Pulse Rate SpO2 Oximeter Blood Oxygen Carry Case Neck Wrist Cord

Soft Rubber cover silicone case protector for CMS50DL/50D/D+/50DL pulse oximeter
By clicking "Accept All Cookies", you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts.
Accept All Cookies