US $780
| Condition | Used
:
An item that has been used previously. The item may have some signs of cosmetic wear, but is fully operational and functions as intended. This item may be a floor model or store return that has been used. See the seller’s listing for full details and description of any imperfections.
|
| Seller Notes | “EXCELLENT CONDITION” |
Directions
Similar products from Other Equipment for Particular Medical Areas
NEW DEVILBISS Medicinal Clear Glass Atomizer Model 15

DEVILBISS Medicinal Clear Glass Atomizer Model 15 Good Condition

2001 VASOSOLUTIONS VASOMEDICAL EECP-MC2 ENHANCED EXTERNAL COUNTERPULSATION SYS.

Reusable mouthpieces for CONTEC Digital Spirometer CMS-SP10\SP10W,Pack of 50pcs
2005 Siemens Sonoline G20 Ultrasound System W/ C5-2 Convex Transducer & Printer
Philips Agilent HP Sonos 5500 Ultrasound W/ S4 / S12 / 11-3L Probe & Footswitch
DermaMed Solutions MegaPeel Platinum Series microdermabrasion system
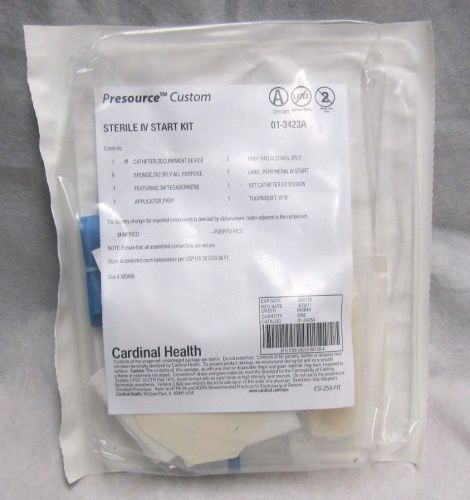
Cardinal Health Presource Custom Sterile IV Starter Kit 01-3423A Exp. : 03-2014

RN+Systems 30 Day Wheelchair Sensor #BPP-30WC Lot Of 5

Refurbished GE LOGIQ 9 W/ 7L Linear 10S Sector i12L Linear & 3.5C Convex Probes

?THE PHYSIOLOGY COLORING BOOK:MEDICAL,OSTEOPATHY LEARN ANATOMY,ILLUSTRATED STUDY
?THE ANATOMY COLORING BOOK:MEDICAL,OSTEOPATHY LEARN PHYSIOLOGY,ILLUSTRATED STUDY

ESSENTIALS OF PHARMACOLOGY FOR HEALTH OCCUPATIONS:RX BOOK+CD PHYSIOLOGY,PHARMACY

STUDENT WORK BOOK:MEDICAL ASSISTING: ADMINISTRATIVE CLINICAL PROCEDURES:ANATOMY

Reusable mouthpieces for CONTEC Digital Spirometer CMS-SP10\SP10W,Pack of 100pcs

TBJ STAINLESS STEEL FORMALIN DISPENSER SINK HOOD ENCLOSURE CABINET WORKSTATION

Cooper Surgical Frigitronics Cryo-Plus Cervix Probe 2442, New

RCI Conchatherm III Servo Controlled Heater 380-80R

Cryomedics Cryosurgical System KR Med MT 600
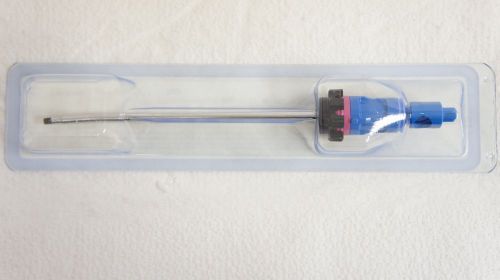
Smith&nephew 7205322 Orbit Razorcut Blade 4.5mm ~ Lot of 4
People who viewed this item also vieved
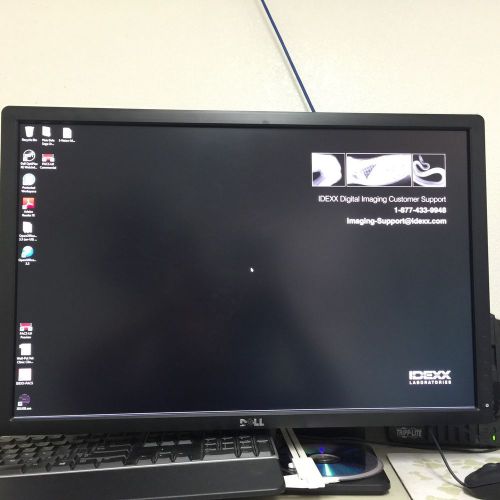
IDEXX Vision CR Digital Radiology System
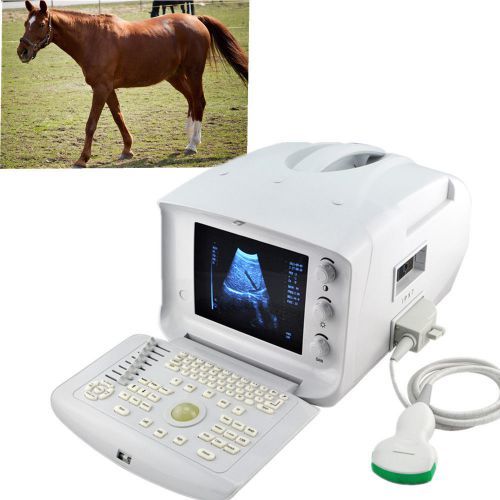
FDA CE Veterianry VET Ultrasound Scanner machine W Convex Probe high quality

FDA Veterianry Ultrasound Scanner machine Micro Convex Probe cardiac abdomen 3D

PEGASYS ETHICON ENDO-SURGERY CAT # RF111 GENERATOR
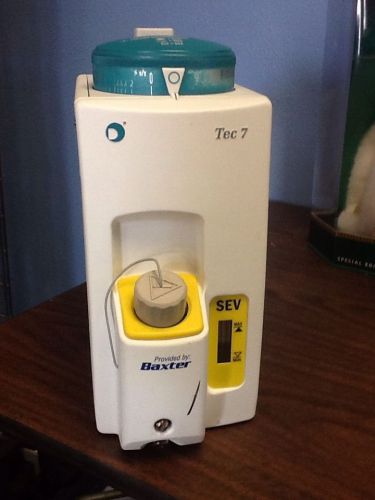
DATEX OHMEDA Anesthesia Isoflurane Vaporizer Tec 7 1175-9101-000

Arthrex AR-1510FPR Femoral Right 7mm (Qty 1)

Abdominal Binder for Protection After Surgery and Support the Belly

Huge Lot of Arm & Wrist Splints (58 Boxes!!)

Codman Surgical Spinal Orthopedic 90° Up 3mm Lumbar Kerrison Rongeur 53-1415
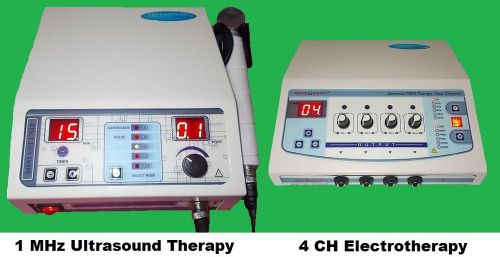
LOT OF 2x ULTRASOUND 1MHz & ELECTROTHERAPY 4CH PAIN REST DEEP HEAT TREATMENT UA6

Thera-Band Pro Series SCP Ball (Retail Pack), Green, 65cm
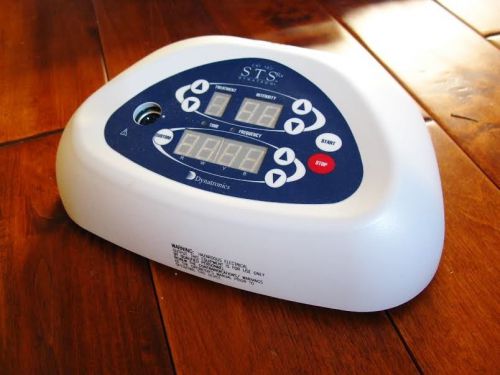
Dynatron Dynatronics Ultrasound Muscle Stimulator Chiropractic Physical Therapy

NELLCOR PURITAN BENNETT STARTRACK Graphics Monitor/ TESTED
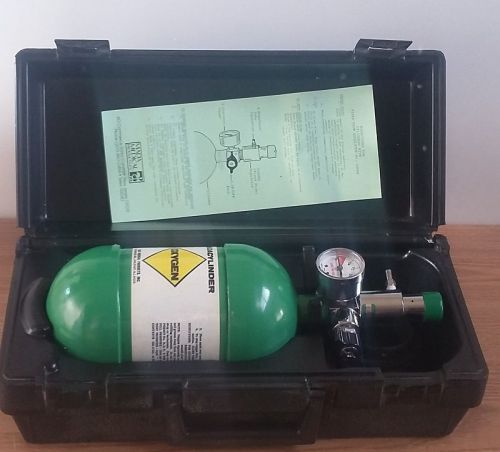
Madacylinder Oxygen Tank With Valve- Empty

Broken Used Nebulizer Repairment
Chiropractor Handmade OOAK Woodburned Sign Plaque Office Decor Chiropractic

Chiropractic Physical Therapy IASTM Soft Tissue Guasha Crossfit Graston Massage

Chiropractic Adjustment Physical Therapy Treatment & Massage Table Power Hi-Lo
By clicking "Accept All Cookies", you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts.
Accept All Cookies