US $99.00
Directions
Similar products from Filters & Filtrarion Accessories

Supelco 24564 Big SupelCarb HC 750cc 1/4 Inch Sealed Package New In Box
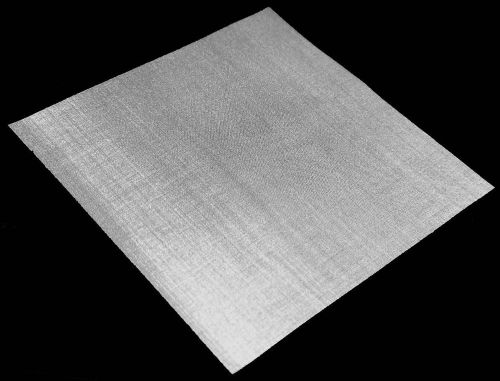
EHpro Stainless Steel 316 #400 Mesh Filtration 105mm 105mm Woven wire 4'' *4'' 3

VWR Valuprep Sterile Syringe Filters, 28145-470, 0.2 uM, Cellulose Acetate, 19ct

MILLIPORE ION-EX CARTRIDGE - TWO NEW IN BOX- F1EA65917 -12"

Varian/Agilent MEGA BE C18 Flash Cartridge SPE, 20 g, 60 mL, 16 pk, New
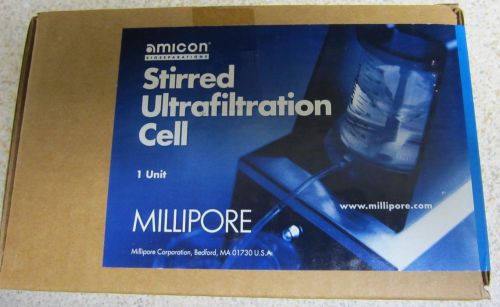
Millipore Amicon Stirred Ultrafiltration Cell 10mL Model 8010
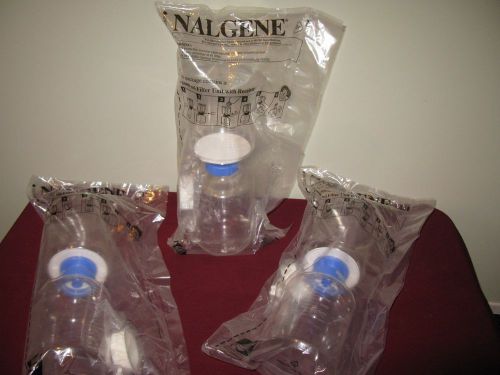
Nalgene 1,000 mL Sterile Filter System individually wrapped

Sartorius Sartofluor GA Cartridge Filter 0.2 µm 5182507T0

Sartorius Sartopure® GF Plus 1.2 micron filter MidiCaps 5555303P0--SS--V 2/pack

Sartorius Sartobran® P 0.45 + 0.2 mic filter MidiCaps® 5235307H0--SS--V 2/Pack

Lab New Old Stock Laboratory Buehler Carbimet Paper Sheets 9x11 (23x28cm) #1

200mm Porcelain Buchner Funnel Xlarge/We Sell 7 Sizes

150mm Porcelain Buchner Funnel LARGE/We Sell 7 Sizes

Sartorius Stedim Sartoguard NF Filter

MILLIPORE TFF Labscale Tangential Ultra Filtration Multi Manifold Kit XXMULTIMN

^^ ALCATEL PUMP FILTER HOUSING? 15" LONG / 6" DIAMETER

PALL Emflon II Gas Filter 0.2um AB05V0022PVJ with Certificate of Test

Millipore Filters MF Support Pads AP10 37mm White CAT NO. AP10037X

1,000 pack PALL Life Sciences 13mm Syringe Filter 0.2u Ion Chrom Acrodisc

Porcelain Buchner Funnel 80mm Filtration Filter New
People who viewed this item also vieved

VWR Basic Protection LabCoats 414004-356 Blue XL with 3 Pockets 30 PCS In Case

Buchner Fritted Filter Funnel Medium 150ml Pyrex Glass

100ml DOERR Graduated Glass Measuring Cylinder

Optical Pliers _ Double Nylon Pliers

Mixed lot Biology Dissection Set-Dissecting Tool Kit Scapel adams germany italy

5 Swagelok SS-600-R-12 Stainless Tube Fitting Reducer, 3/8" Fitting 3/4" Tube

1/2" Sanitary Stainless Tri Clamp Tri Clover 6-Position Manifold 316L 0.5"

Orochem Cat # OT100PR12-10 96 Well, 1.2 mL 8/Case
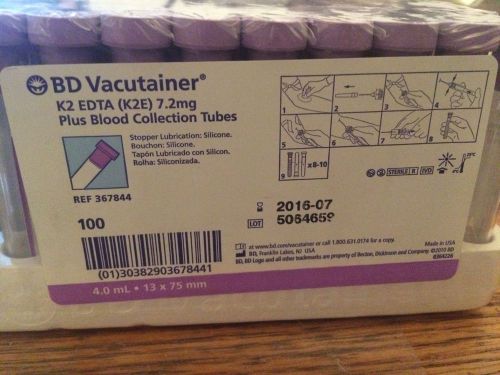
BD VACUTAINER 100 BLOOD COLLECTION TUBES K2 EDTA LAV #367844

COSTOR 6-WELLCell Culture Cluster w/Lid part3516. Sealed in package qly22

COSTOR 24-WELLCell Culture Cluster w/Lid part3526. Sealed in package qly22
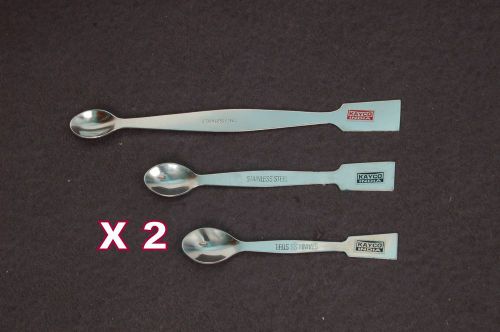
6 Pcs SPATULA STAINLESS STEEL-Set of 3 x 2 Lab Equipment Medical/General /Pharma
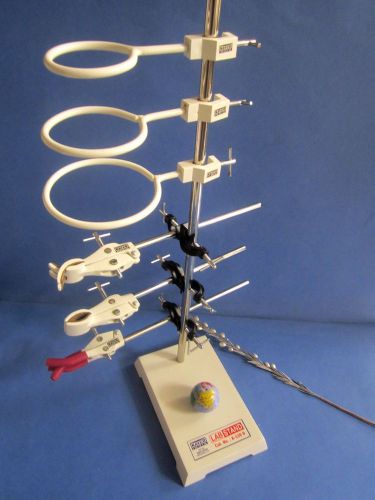
New Heavy Lab Stand 8"x5"x24"-10 Pc Tool Kit A1 w/ Clamps, Retort Holders Etc.

Quidel Sofia Installation Pack 20230

Brookfield LV Spindle Set Kit Digital Viscometers

3 Rainin EDP2 Motorized Dispensing Pipettes (10-1000 ul)

3 Used Oxford Pipettes (2 – 200 ul)

1,5-Diphenylcarbazide, Analytical Reagent ACS, 50g

Hanna Instruments HI 70631 Electrode Cleaning Solution

C & A Scientific Extraordinary Ordinary Prepared Slide Set

100pc Microscope Slide Storage Box, Black
By clicking "Accept All Cookies", you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts.
Accept All Cookies