US $99.00
Directions
Similar products from Filters & Filtrarion Accessories

Sartorius Stedim Sartofluor GA Cartridge Filter, 0,2µm, 5182507T0----GA SEALED!

Case Of 9 VWR 87006-062 Vacuum Filtration Systems, PES Membrane, Sterile SEALED

Supelco 24564 Big SupelCarb HC 750cc 1/4 Inch Sealed Package New In Box
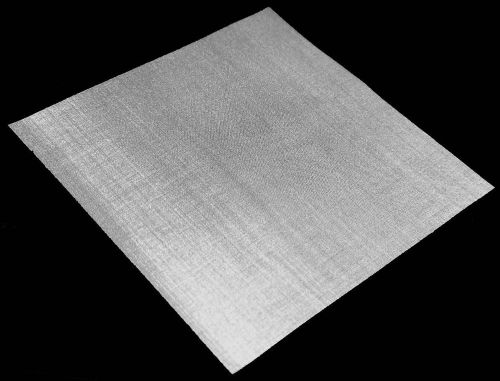
EHpro Stainless Steel 316 #400 Mesh Filtration 105mm 105mm Woven wire 4'' *4'' 3

VWR Valuprep Sterile Syringe Filters, 28145-470, 0.2 uM, Cellulose Acetate, 19ct

MILLIPORE ION-EX CARTRIDGE - TWO NEW IN BOX- F1EA65917 -12"

Varian/Agilent MEGA BE C18 Flash Cartridge SPE, 20 g, 60 mL, 16 pk, New
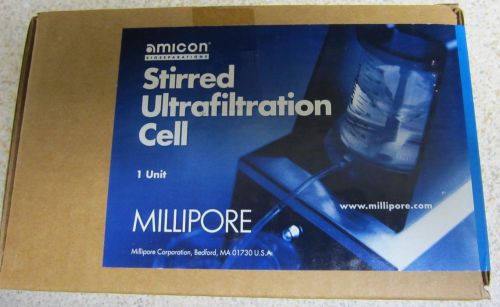
Millipore Amicon Stirred Ultrafiltration Cell 10mL Model 8010
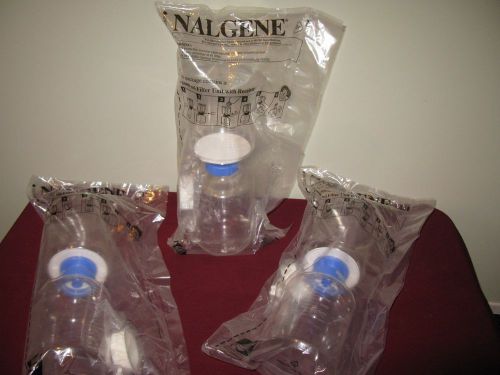
Nalgene 1,000 mL Sterile Filter System individually wrapped

Sartorius Sartofluor GA Cartridge Filter 0.2 µm 5182507T0

Sartorius Sartobran® P 0.45 + 0.2 mic filter MidiCaps® 5235307H0--SS--V 2/Pack

Sartorius Sartobran® P 0.65 + 0.45 mic filter MidiCaps® 5235306D8--OO--A 4/Pack

Lab New Old Stock Laboratory Buehler Carbimet Paper Sheets 9x11 (23x28cm) #1

200mm Porcelain Buchner Funnel Xlarge/We Sell 7 Sizes

150mm Porcelain Buchner Funnel LARGE/We Sell 7 Sizes

Sartorius Stedim Sartoguard NF Filter

MILLIPORE TFF Labscale Tangential Ultra Filtration Multi Manifold Kit XXMULTIMN

^^ ALCATEL PUMP FILTER HOUSING? 15" LONG / 6" DIAMETER

PALL Emflon II Gas Filter 0.2um AB05V0022PVJ with Certificate of Test

Millipore Filters MF Support Pads AP10 37mm White CAT NO. AP10037X
People who viewed this item also vieved
Autoclave Indicator Tape, Stripes, 3/4 x 2160", 1 each

GUARDIAN G1994 SAFETY STATION WITH EYE / FACE WASH NEW IN BOX

Lot of 2 new Kimax 300ML, 24/40 Round Bottom Boiling Flask old stock 25285 lab

QTY 5-Kimax Kimble Chase WIDE MOUTH ERLENMEYER FLASK 26650-1000 ml lab glassware

Lot of 13 Plastipak BD Syringe 20 CC
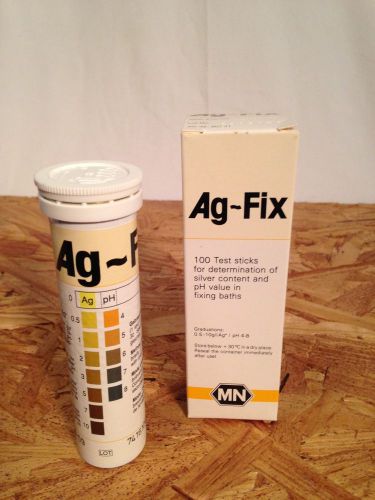
Silver Test Sticks Package of 100 AG-FIX Determine Silver Content in Liquid

LOT OF 316L SS SANITARY FITTINGS CLAMPS ELBOW STRAIGHT FLANGED (S24-2-20E)

Swagelok 1.25" Stainless Steel Front Ferrule SS-2003-1 Sevral Avail New

Kimtech Science Kimwipes Delicate Task Wipers 11.8"x11.8" 196 ct. 34133

1000 FISHER CIRCLES REDI-WEIGH WEIGHING PAPERS 3 1/8" DIAMETER LAB LABORATORY

Globe Scientific 601608-10 LDPE Wide Mouth Flexible Round Bottle with
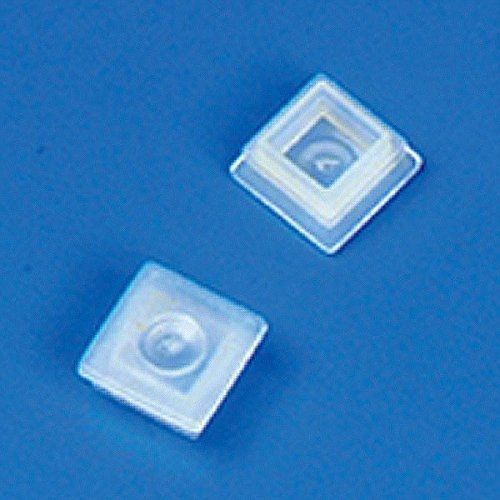
Globe Scientific 111167 Polypropylene Cap and Plug for Square Spectrophotometer
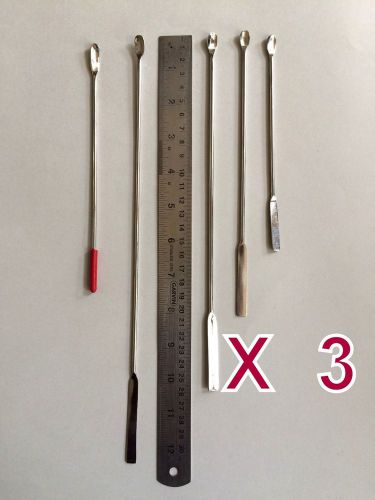
KAYCO 15 Pieces MICRO-SPATULA STAINLESS STEEL 5Pc x 3 - Medical/General Lab Aid
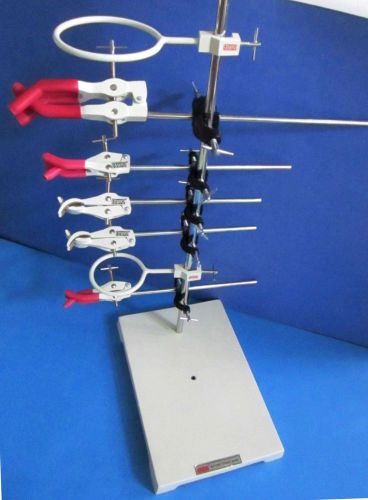
EXtra Heavy 12" x 8" x 36" Lab Stand w/ Stainless Steel Rod & Clamps Etc kit
Agilent 9910078300 - KIT CARY 13 WL DRIVE and LUBRICATION

480 Fisherbrand Gel Loading Pipet Tips 05-541-9 5 Racks of 96 Each
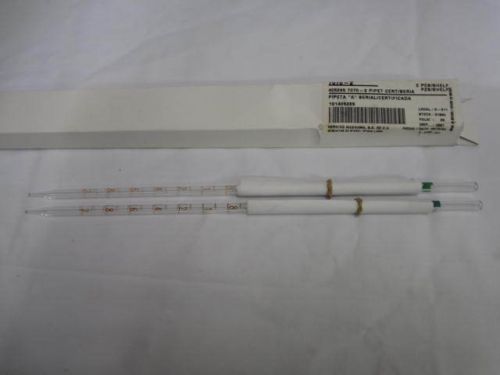
Lot of 2 Corning 7070-2 Borosilicate Glass Pipets Graduated 2ml New NOS

Molybdenum (VI) oxide, pure reagent 100g, CAS 1313-27-5

100 grams (3.52 oz) High Purity 99.9% 5N Pure Selenium Element Pellets Beads

Lot of HS VWR Fisher Scientific Microscope Case Slide Holders 48443-006 3-445

CORNING 2935 223 COVER GLASS 22 X 30MM 5-CASES NEW $49
By clicking "Accept All Cookies", you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts.
Accept All Cookies