US $180
Directions
Similar products from Chemical Supplies

Chromium nitrate, >97%, Extra pure, 500 gm

Cupric Acetate monohydrate , >98%, Extra pure, 500 gm

16940-66-2 Sodium BOROHYDRIDE 99,99% DRY granular 1000g (Na- tetrahydroborate)

Wallach Ferric Subsulfate Aqueous Solution (12/Box)
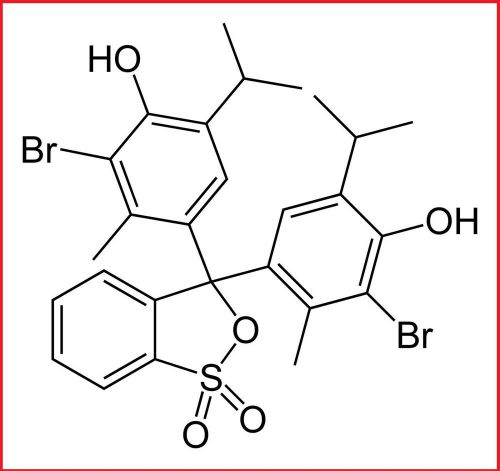
Bromothymol blue 0.1% alcohol solution 30ml

Paraformaldehyde Lab chemical 300g
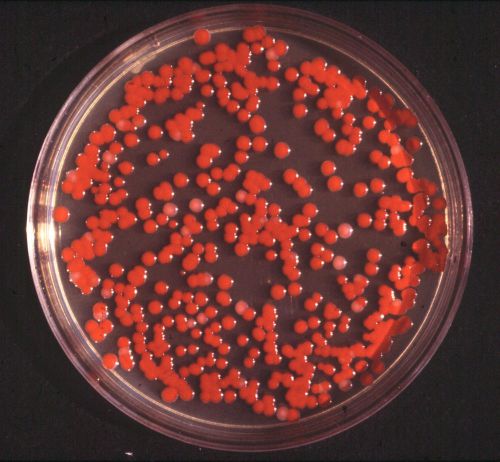
Mueller-Hinton agar dehydrated media 50g

4 Pounds Aluminum Powder - 5 micron Super Fine

C14-16 Alpha Olefin Sulfonate 40% Solution Surfactant 5 gallon

Sodium Hexametaphosphate SHMP GRAHAM'S SALT Calgon 10 Lb
1-Iodobutane, Butyl iodide, 99%, 50ml
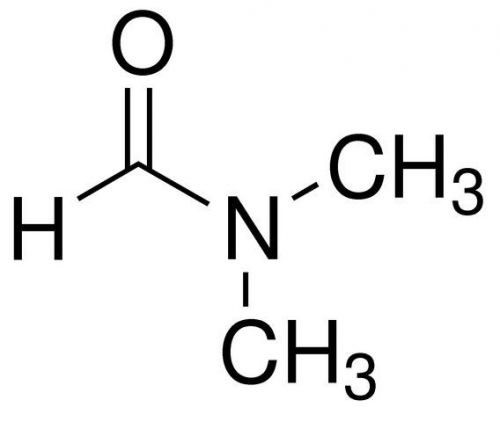
Dimethylformamide, N,N-Dimethylformamide, DMF, 99%, 100ml
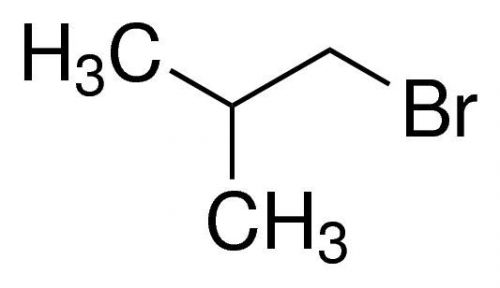
1-Bromo-2-methylpropane, Isobutyl bromide, 99%, 50ml
1-Bromobutane, Butyl bromide, 99.0+%, 50ml
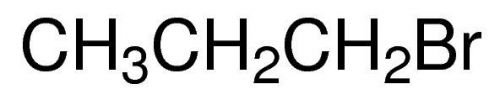
1-Bromopropane, Propyl bromide, 99%, 50ml

Extremely Pure / Fine Silver metal powder 90 grams / ~3 OZ MADE / SHIPS From USA

Extremely Pure / Fine Aluminum Powder 20 lbs / MADE and SHIPS FROM USA

1Rolls 99.95% 25g 70ft Magnesium Ribbon High Purity Lab Chemicals New
People who viewed this item also vieved
Firm Useful 10X Sterile Plastic Petri Dishes For LB Plate Bacteria 55x15mm HGCA

Ampac Flexibles Polyester Pouches, Ampac 506B-500 Heavy-Duty, 4.5 Mil
EHpro Stainless Steel 316 #400 Mesh Filtration 105mm 105mm Woven wire 4'' *4'' 3

Supelco 24564 Big SupelCarb HC 750cc 1/4 Inch Sealed Package New In Box

AGILENT 0905-1463 NW10/16 TRAPPED O-RING SEAL - NEW SURPLUS

KECK CLIPS, 10/30 10/18, LOT OF 20 Clamps, Chemglass, NIB, Sealed, CG-145-01

New Disposable Culture Tubes VWR Cat # 60825-673 18mm x 150mm Qty 125

Kimble® KIMAX PTFE Color-Coded Stopper Size 29 Set of 3
8 Funnels Plastic Variety Sizes All-Purpose for Perfume & Fragrance Decanting
5.11 TMT L2 Flashlight Black (1EA)
5.11 LiIon 18650 Rechg Batt Pk Black (2EA)

Thermo Slide-A-Lyzer Dialysis Cassette #66830
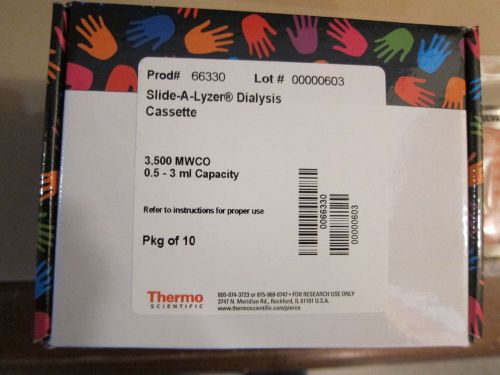
Thermo Slide-A-Lyzer Dialysis Cassette #66330

Viaflo Electronic Pipette 4633, 12-Channel 10-300uL w/ Stand & Charger *Parts*

Rainin EDP-3 Plus Pipettes Lot of (3) w/ Charging Stand & Charger 3

Nalgene 20L Clear Polycarbonate Graduated Rectangular Carboy w/ Metal Handle

Plastic, Graduated, Polypropylene Beaker w Pour Spout : 1000ml

Aqua Colorfrost Microscope Slides 9951A-006

Shandon COLORFROST PLUS Microscope Slides 100 pack
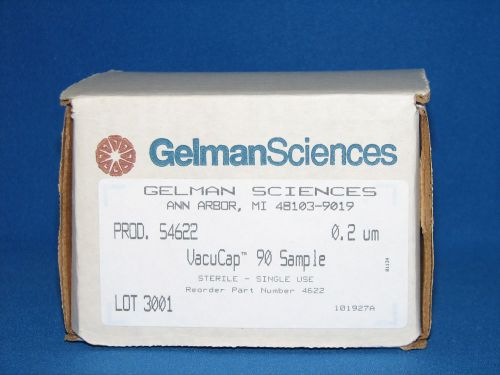
Gelman Sciences VacuCap 90 Sample Catalog # 54622 0.2 um.

NALGENE 6152-0500 Polypropylene Y-Type Tubing Connector, 1/2" Tubing ID (Pack of
By clicking "Accept All Cookies", you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts.
Accept All Cookies