US $99.00
Directions
Similar products from Filters & Filtrarion Accessories

Case Of 9 VWR 87006-062 Vacuum Filtration Systems, PES Membrane, Sterile SEALED

Supelco 24564 Big SupelCarb HC 750cc 1/4 Inch Sealed Package New In Box
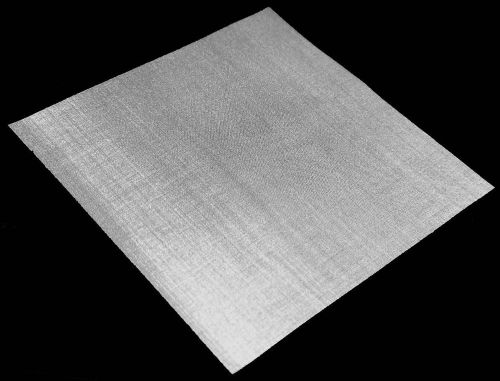
EHpro Stainless Steel 316 #400 Mesh Filtration 105mm 105mm Woven wire 4'' *4'' 3

VWR Valuprep Sterile Syringe Filters, 28145-470, 0.2 uM, Cellulose Acetate, 19ct

MILLIPORE ION-EX CARTRIDGE - TWO NEW IN BOX- F1EA65917 -12"

Varian/Agilent MEGA BE C18 Flash Cartridge SPE, 20 g, 60 mL, 16 pk, New
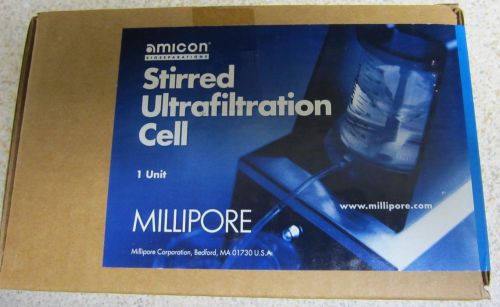
Millipore Amicon Stirred Ultrafiltration Cell 10mL Model 8010
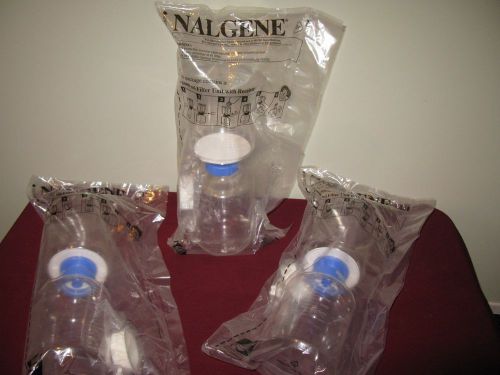
Nalgene 1,000 mL Sterile Filter System individually wrapped

Sartorius Sartofluor GA Cartridge Filter 0.2 µm 5182507T0

Sartorius Sartopure® GF Plus 1.2 micron filter MidiCaps 5555303P0--SS--V 2/pack

Sartorius Sartobran® P 0.65 + 0.45 mic filter MidiCaps® 5235306D8--OO--A 4/Pack

Lab New Old Stock Laboratory Buehler Carbimet Paper Sheets 9x11 (23x28cm) #1

200mm Porcelain Buchner Funnel Xlarge/We Sell 7 Sizes

150mm Porcelain Buchner Funnel LARGE/We Sell 7 Sizes

Sartorius Stedim Sartoguard NF Filter

MILLIPORE TFF Labscale Tangential Ultra Filtration Multi Manifold Kit XXMULTIMN

^^ ALCATEL PUMP FILTER HOUSING? 15" LONG / 6" DIAMETER

PALL Emflon II Gas Filter 0.2um AB05V0022PVJ with Certificate of Test

Millipore Filters MF Support Pads AP10 37mm White CAT NO. AP10037X

1,000 pack PALL Life Sciences 13mm Syringe Filter 0.2u Ion Chrom Acrodisc
People who viewed this item also vieved

GUARDIAN G1994 SAFETY STATION WITH EYE / FACE WASH NEW IN BOX

KIMBLE KIMAX 5pc. Flask set 50ml-1,000ml

Buchner Fritted Filter Funnel Medium 150ml Pyrex Glass
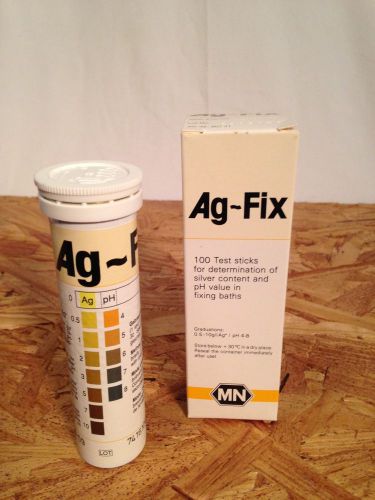
Silver Test Sticks Package of 100 AG-FIX Determine Silver Content in Liquid

Optical Pliers _ A Set of Pliers_Rimless Bushing Removing and Mounting Pliers

Lot of 10: Swagelok 1/2" Stainless Steel Ferrule Set SS-810-SET Sevral Avail New

5 Swagelok SS-600-R-12 Stainless Tube Fitting Reducer, 3/8" Fitting 3/4" Tube

1000 FISHER CIRCLES REDI-WEIGH WEIGHING PAPERS 3 1/8" DIAMETER LAB LABORATORY

Orochem Cat # OT100PR12-10 96 Well, 1.2 mL 8/Case
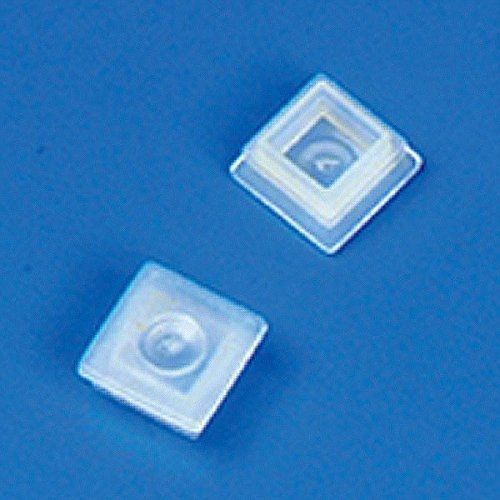
Globe Scientific 111167 Polypropylene Cap and Plug for Square Spectrophotometer

COSTOR 6-WELLCell Culture Cluster w/Lid part3516. Sealed in package qly22
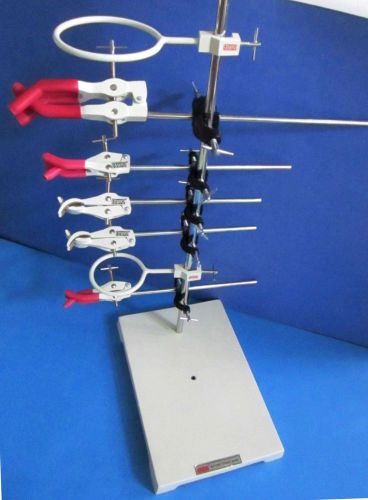
EXtra Heavy 12" x 8" x 36" Lab Stand w/ Stainless Steel Rod & Clamps Etc kit
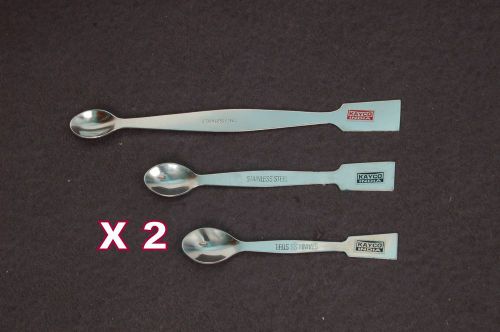
6 Pcs SPATULA STAINLESS STEEL-Set of 3 x 2 Lab Equipment Medical/General /Pharma

Quidel Sofia Installation Pack 20230

Package of 10 Scigen Neutra-Pads 4295

Fisher Scientific Laboratory Counter W/2 Counting Units NOS

Dimethylglyoxime, >99%, Analytical Reagent ACS, 50 gm

1,5-Diphenylcarbazide, Analytical Reagent ACS, 50g

CORNING 2935 223 COVER GLASS 22 X 30MM 5-CASES NEW $49

C & A Scientific Extraordinary Ordinary Prepared Slide Set
By clicking "Accept All Cookies", you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts.
Accept All Cookies